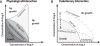Multidrug evolutionary strategies to reverse antibiotic resistance - PubMed (original) (raw)
Review
Multidrug evolutionary strategies to reverse antibiotic resistance
Michael Baym et al. Science. 2016.
Abstract
Antibiotic treatment has two conflicting effects: the desired, immediate effect of inhibiting bacterial growth and the undesired, long-term effect of promoting the evolution of resistance. Although these contrasting outcomes seem inextricably linked, recent work has revealed several ways by which antibiotics can be combined to inhibit bacterial growth while, counterintuitively, selecting against resistant mutants. Decoupling treatment efficacy from the risk of resistance can be achieved by exploiting specific interactions between drugs, and the ways in which resistance mutations to a given drug can modulate these interactions or increase the sensitivity of the bacteria to other compounds. Although their practical application requires much further development and validation, and relies on advances in genomic diagnostics, these discoveries suggest novel paradigms that may restrict or even reverse the evolution of resistance.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Fig. 1. Physiological interactions and cross-resistance
(A) Isoboles of minimum inhibitory concentration (MIC) are shown in the two-drug concentration space for different drug interactions. The MIC of each drug alone occurs where the isobole intersects each drug axis. When the effect of the two drugs is equal to the effect expected when combining two identical drugs, the shape of the MIC line is linear and the drugs are said to be noninteracting (104). Synergistic drugs require less-than-expected concentrations, corresponding to a concave MIC line, whereas antagonistic interactions require higher drug concentrations, producing a convex line. Finally, drug interactions are suppressive when their effect in combination is less than that of one of the drugs alone, appearing as a nonmonotonic isobole. (B) Cross-resistance and collateral sensitivity: A mutation or acquired gene conferring resistance to drug A can also increase resistance (positive cross-resistance) or decrease resistance (negative cross-resistance or collateral sensitivity) to drug B without otherwise changing the shape of the interaction.
Fig. 2. Selection inversion approaches and potential strategies
(A) In typical drug interactions, the region of growth of a single-drug–resistant mutant (e.g., A-resistant, dashed area) completely covers the region of growth of the drug-sensitive wild type (gray area), and the mutant therefore always outcompetes the wild type. (B to D) There are three principal ways for establishing a concentration regime (*) that selects against resistance: (B) When drug A suppresses drug B, the MIC isobole is nonmonotonic, and so scaling it along the A axis because of resistance leaves a selection-inverting regime. (C) An antagonistic interaction can become synergistic with the acquisition of resistance, making the mutant more sensitive to the combination. (D) Collateral sensitivity, when the MIC of drug B decreases as a result of resistance to drug A, allowing selection against resistance even in the absence of A. (E) Using selection inversion approaches on a nonresistant population can decrease the probability of resistance evolution and make long-term therapy more likely to succeed. (F) Selection against resistance can also be used as part of a two-phase strategy against a population with resistant mutants. The drug-resistant mutants are selected out of the population in the first phase, allowing a previously ineffective antibiotic to be used in the second.
Fig. 3. The efficacy and potential failure of cycling collaterally sensitive antibiotics
(A) Fitness landscapes in collaterally sensitive antibiotics. Genotypes that are resistant to drug A or drug B appear as fitness peaks when the environment contains the drug to which they are resistant but as fitness valleys in the other drug treatment. In principle, alternating the drugs can lead to a cycle of evolution switching between these genotypes (solid arrows). However, doubly resistant mutants can evade this trap (dashed arrows). (B) Two possible evolutionary trajectories in the MICs of component drugs in antibiotic cycling. Ideally, resistance will alternate between two states (solid arrows). However, repeated accumulation of resistance mutations can also create double-resistance, even in the case where each individual mutation induces collateral sensitivity (dashed arrow).
Similar articles
- Using experimental evolution to identify druggable targets that could inhibit the evolution of antimicrobial resistance.
Mehta HH, Prater AG, Shamoo Y. Mehta HH, et al. J Antibiot (Tokyo). 2018 Feb;71(2):279-286. doi: 10.1038/ja.2017.108. Epub 2017 Sep 20. J Antibiot (Tokyo). 2018. PMID: 28928474 Free PMC article. - European Society for Evolutionary Biology meeting. Tracing the common roots of antibiotic resistance.
Pennisi E. Pennisi E. Science. 2011 Sep 16;333(6049):1562-3. doi: 10.1126/science.333.6049.1562-b. Science. 2011. PMID: 21921169 No abstract available. - Multi-step vs. single-step resistance evolution under different drugs, pharmacokinetics, and treatment regimens.
Igler C, Rolff J, Regoes R. Igler C, et al. Elife. 2021 May 18;10:e64116. doi: 10.7554/eLife.64116. Elife. 2021. PMID: 34001313 Free PMC article. - Collateral sensitivity of antibiotic-resistant microbes.
Pál C, Papp B, Lázár V. Pál C, et al. Trends Microbiol. 2015 Jul;23(7):401-7. doi: 10.1016/j.tim.2015.02.009. Epub 2015 Mar 25. Trends Microbiol. 2015. PMID: 25818802 Free PMC article. Review. - Evolutionary Trajectories to Antibiotic Resistance.
Hughes D, Andersson DI. Hughes D, et al. Annu Rev Microbiol. 2017 Sep 8;71:579-596. doi: 10.1146/annurev-micro-090816-093813. Epub 2017 Jul 11. Annu Rev Microbiol. 2017. PMID: 28697667 Review.
Cited by
- Using tea nanoclusters as β-lactamase inhibitors to cure multidrug-resistant bacterial pneumonia: A promising therapeutic strategy by Chinese materioherbology.
Zhou Z, Li J, Tan L, Liu X, Zheng Y, Cui Z, Li C, Yeung KWK, Li Z, Liang Y, Zhu S, Wu S. Zhou Z, et al. Fundam Res. 2021 Nov 25;2(3):496-504. doi: 10.1016/j.fmre.2021.11.019. eCollection 2022 May. Fundam Res. 2021. PMID: 38933406 Free PMC article. - Extent and context dependence of pleiotropy revealed by high-throughput single-cell phenotyping.
Geiler-Samerotte KA, Li S, Lazaris C, Taylor A, Ziv N, Ramjeawan C, Paaby AB, Siegal ML. Geiler-Samerotte KA, et al. PLoS Biol. 2020 Aug 17;18(8):e3000836. doi: 10.1371/journal.pbio.3000836. eCollection 2020 Aug. PLoS Biol. 2020. PMID: 32804946 Free PMC article. - Coumarin derivatives ameliorate the intestinal inflammation and pathogenic gut microbiome changes in the model of infectious colitis through antibacterial activity.
Jung HS, Park YJ, Gu BH, Han G, Ji W, Hwang SM, Kim M. Jung HS, et al. Front Cell Infect Microbiol. 2024 Jul 15;14:1362773. doi: 10.3389/fcimb.2024.1362773. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39081865 Free PMC article. - Water-soluble PANI:PSS designed for spontaneous non-disruptive membrane penetration and direct intracellular photothermal damage on bacteria.
Tang H, Liu Y, Li B, Shang B, Yang J, Zhang C, Yang L, Chen K, Wang W, Liu J. Tang H, et al. Bioact Mater. 2021 May 23;6(12):4758-4771. doi: 10.1016/j.bioactmat.2021.05.019. eCollection 2021 Dec. Bioact Mater. 2021. PMID: 34136724 Free PMC article. - Reversion of Ceftazidime Resistance in Pseudomonas aeruginosa under Clinical Setting.
Liu Q, Yin L, Zhang X, Zhu G, Liu H, Bai F, Cheng Z, Wu W, Jin Y. Liu Q, et al. Microorganisms. 2022 Dec 2;10(12):2395. doi: 10.3390/microorganisms10122395. Microorganisms. 2022. PMID: 36557649 Free PMC article.
References
- Abraham EP, et al. Further observations on penicillin. Lancet. 1941;238:177–189.
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. 2013 www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf.
- Laxminarayan R. Antibiotic effectiveness: Balancing conservation against innovation. Science. 2014;345:1299–1301. pmid: 25214620. - PubMed
- President’s Council of Advisors on Science, Technology, “Report to the President on Combating Antibiotic Resistance”. Executive Office of the President; 2014. pp. 1–78.
- World Health Organization. Antimicrobial resistance: Global report on surveillance. World Health Organization; Geneva, Switzerland: 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical