Hyperthermia-induced radiosensitization in CHO wild-type, NHEJ repair mutant and HR repair mutant following proton and carbon-ion exposure - PubMed (original) (raw)
Hyperthermia-induced radiosensitization in CHO wild-type, NHEJ repair mutant and HR repair mutant following proton and carbon-ion exposure
Junko Maeda et al. Oncol Lett. 2015 Nov.
Abstract
The DNA repair mechanisms involved in hyperthermia-induced radiosensitization with proton and carbon ion radiation exposure were investigated in the present study. In a previous study, Chinese hamster ovary (CHO) cells were exposed to low linear energy transfer (LET) photon radiation. These cells can be sensitized by hyperthermia as a result of inhibition of homologous recombination (HR) repair. The present study used wild-type, non-homologous end joining (NHEJ) and HR repair-deficient CHO cells to define the contributions of each repair pathway to cellular lethality following hyperthermia-induced hadron radiation sensitization. The cells were exposed to ionizing radiation, followed by hyperthermia treatment (42.5°C for 1 h). Hyperthermia-induced radiosensitization was determined by the colony formation assay and thermal enhancement ratio. HR repair-deficient cells exhibited no hyper-sensitization to X-rays, protons, or low and high LET carbon ions when combined with hyperthermia. Wild-type and NHEJ repair-deficient cells exhibited significant hyperthermia-induced sensitization to low LET photon and hadron radiation. Hyperthermia-induced sensitization to high LET carbon-ion radiation was less than at low LET radiation. Relative biological effectiveness (RBE) between radiation alone and radiation combined with hyperthermia cell groups was not significantly different in any of the cell lines, with the exception of wild-type cells exposed to high LET radiation, which exhibited a lower RBE in the combined group. The present study investigated additional cell lines to confirm the lower RBE observed in DNA repair-deficient cell lines. These findings suggested that hyperthermia-induced hyper-sensitization to hadron radiation is also dependent on inhibition of HR repair, as was observed with photon radiation in a previous study.
Keywords: DNA repair; hadron radiation; hyperthermia.
Figures
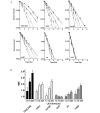
Figure 1.
Cell survival curves for CHO wild-type, V3, and 51D1 cells exposed to (A) X-ray, (B) protons, (C) carbon 13 keV/µm, (D) carbon 30 keV/µm or (E) carbon 70 keV/µm. Solid lines are the control group (radiation alone). Dashed lines are the combined group (radiation with post-irradiation hyperthermia). Error bars are standard error of the mean values of at least three independent experiments. Trendlines were constructed using GraphPad Prism 6 with linear quadratic regression. *P<0.05. CHO, Chinese hamster ovary.
Figure 2.
(A) D10 values of the control group (radiation alone, closed circles) and combined group (radiation combined with post-irradiation hyperthermia, open circles). (B) TER values of the control and combined groups. (C) RBE values of the control and combined groups. *P<0.05. CHO, Chinese hamster ovary; LET, linear energy transfer., RBE, relative biological effectiveness; TER, thermal enhancement ratio; D10, radiation dose to achieve 10% cell survival.
Figure 3.
(A) Cell survival curves for CHO wild-type cells, NHEJ repair-deficient cells and HR repair-deficient cells. Closed circles indicate X-rays, closed triangles indicate carbon ion LET 13 keV/µm, open circles indicate carbon ion LET 70 keV/µm, and open squares indicate iron ion LET 200 keV/µm. Error bars are the standard error of the mean values of at least three independent experiments. Lines were constructed using GraphPad Prism 6 with linear quadratic regression. (B) RBE values of CHO wild-type, xrs5, V3, irs20, 51D1, and irs1SF cells. CHO, chinese hamster ovary NHEJ, non-homologous end joining; HR, homologous recombination; LET, linear energy transfer; RBE, relative biological effectiveness.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources