FGF21 Regulates Sweet and Alcohol Preference - PubMed (original) (raw)
. 2016 Feb 9;23(2):344-9.
doi: 10.1016/j.cmet.2015.12.008. Epub 2015 Dec 24.
Bryn M Owen 2, Parkyong Song 2, Genaro Hernandez 2, Yuan Zhang 2, Yingjiang Zhou 1, William T Scott 2, Bhavna Paratala 3, Tod Turner 1, Andrew Smith 3, Barbara Bernardo 3, Christian P Müller 4, Hao Tang 5, David J Mangelsdorf 6, Bryan Goodwin 1, Steven A Kliewer 7
Affiliations
- PMID: 26724861
- PMCID: PMC4749404
- DOI: 10.1016/j.cmet.2015.12.008
FGF21 Regulates Sweet and Alcohol Preference
Saswata Talukdar et al. Cell Metab. 2016.
Abstract
Fibroblast growth factor 21 (FGF21) is a hormone induced by various metabolic stresses, including ketogenic and high-carbohydrate diets, that regulates energy homeostasis. In humans, SNPs in and around the FGF21 gene have been associated with macronutrient preference, including carbohydrate, fat, and protein intake. Here we show that FGF21 administration markedly reduces sweet and alcohol preference in mice and sweet preference in cynomolgus monkeys. In mice, these effects require the FGF21 co-receptor β-Klotho in the central nervous system and correlate with reductions in dopamine concentrations in the nucleus accumbens. Since analogs of FGF21 are currently undergoing clinical evaluation for the treatment of obesity and type 2 diabetes, our findings raise the possibility that FGF21 administration could affect nutrient preference and other reward behaviors in humans.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
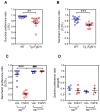
Figure 1. FGF21 decreases sweet preference ratio in mice by acting on the CNS
(A) Two-bottle preference assay in wild-type (WT) and Tg(Fgf21) mice administered water vs. 3% sucrose. Representative 24 hour data from day 2 after initiating the assay are shown as the sucrose preference ratio (sucrose intake volume/total fluid intake volume). n = 10–11/group. (B) Two-bottle preference assay in WT and Tg(Fgf21) mice administered water vs. 0.2% saccharin. Representative 24 hour data from day 2 after initiating the assay are shown. n = 10–11/group. (C) Two-bottle preference assay with water vs. 0.2% saccharin for Klbfl/fl and KlbCamk2a mice administered either FGF21 (1 mg/kg/day) or vehicle. Representative 24 hour data from day 3 after initiating the assay are shown. n = 6–9/group. (D) Two-bottle preference assay with water vs. 2 mg/dl quinine for Klbfl/fl and KlbCamk2a mice administered either FGF21 (1 mg/kg/day) or vehicle. n = 4/group. Values are means ±S.E.M. *, p<0.05; ***, p<0.001; ###, p<0.001 by Student’s _t_-test. See also Figure S1, Table S1 and S2.
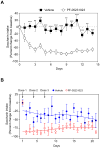
Figure 2. A stable FGF21 analog decreases saccharin preference in mice and monkeys
(A) Two bottle preference assay with 0.1% saccharin in diet-induced obese mice administered either PF-05231023 (10 mg/kg) or vehicle on days 0, 3, 7 and 10. Data are shown as the mean ± S.E.M.; n = 8/group. **p < 0.01, ***p < 0.001 versus vehicle group. (B) Two bottle preference assay with 0.2% saccharin in obese cynomolgus monkeys administered either PF-05231023 (n=8; 10 mg/kg) or vehicle (n=7) on days 1, 4 and 7. Data are presented as mean percentage change in saccharin water intake ± S.E.M. for vehicle-treated (closed blue circles) and PF-05231023-treated (open red circles) monkeys. Solid lines are locally weighted scatterplot smoothing fits to the means of percent change. Mixed effect modeling fitted to these longitudinal data using R, version 3.1.2 (Pinheiro et al., 2013), showed a significant difference (p = 0.003) between groups. Number of days after first treatment, treatment type, and the interaction term between treatment groups and time were specified as fixed effects and monkey labels as a random effect.
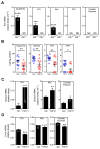
Figure 3. FGF21 affects dopamine signaling
(A) β-Klotho (Klb) mRNA levels in the suprachiasmatic nucleus/paraventricular nucleus (SCN/PVN) region of the hypothalamus, ventral tegmental area (VTA), nucleus accumbens (NAc), medial prefrontal cortex (PFC) and caudate putamen of Klb+/− and _Klb_−/− mice (n = 6/group). Ct values are shown in the bars. ND, not detected. (B) Concentrations of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 3-methoxytyramine (3-MT) in the NAc of mice administered either vehicle or FGF21 for 2 weeks by osmotic minipump (n = 12/group). (C) mRNA levels of dopamine transporter (Slc6a3) or (D) catechol-O-methyl transferase (Comt) in VTA, NAc and caudate putamen of mice administered either vehicle or FGF21 for 2 weeks by osmotic minipump (n = 7–8/group). Ct values are shown. Values are means ±S.E.M. *, p<0.05; **, p<0.01; ***, p<0.001 versus control group by Student’s _t_-test. See also Figure S2.
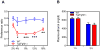
Figure 4. FGF21 decreases alcohol preference
(A) Ethanol preference ratio in wild-type (WT) and Tg(Fgf21) mice at the indicated ethanol concentrations (n = 9/group). (B) Plasma ethanol concentrations in groups of WT and Tg(Fgf21) mice 1 or 3 hours after i.p. injection of ethanol (4g/kg) (n = 4–5/group). Values are means ±S.E.M. *, p<0.05; **, p<0.01; and ***, p<0.001 versus control group by Student’s _t_-test. See also Table S3.
Comment in
- The Sweetest Thing: Regulation of Macronutrient Preference by FGF21.
Adams AC, Gimeno RE. Adams AC, et al. Cell Metab. 2016 Feb 9;23(2):227-8. doi: 10.1016/j.cmet.2016.01.013. Cell Metab. 2016. PMID: 26863484
Similar articles
- FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver.
von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ. von Holstein-Rathlou S, et al. Cell Metab. 2016 Feb 9;23(2):335-43. doi: 10.1016/j.cmet.2015.12.003. Epub 2015 Dec 24. Cell Metab. 2016. PMID: 26724858 Free PMC article. - FGF21 Signals to Glutamatergic Neurons in the Ventromedial Hypothalamus to Suppress Carbohydrate Intake.
Jensen-Cody SO, Flippo KH, Claflin KE, Yavuz Y, Sapouckey SA, Walters GC, Usachev YM, Atasoy D, Gillum MP, Potthoff MJ. Jensen-Cody SO, et al. Cell Metab. 2020 Aug 4;32(2):273-286.e6. doi: 10.1016/j.cmet.2020.06.008. Epub 2020 Jul 7. Cell Metab. 2020. PMID: 32640184 Free PMC article. - FGF21 acts in the brain to drive macronutrient-specific changes in behavioral motivation and brain reward signaling.
Khan MSH, Kim SQ, Ross RC, Corpodean F, Spann RA, Albarado DA, Fernandez-Kim SO, Clarke B, Berthoud HR, Münzberg H, McDougal DH, He Y, Yu S, Albaugh VL, Soto PL, Morrison CD. Khan MSH, et al. Mol Metab. 2025 Jan;91:102068. doi: 10.1016/j.molmet.2024.102068. Epub 2024 Nov 19. Mol Metab. 2025. PMID: 39571902 Free PMC article. - Fibroblast growth factor 21: an endocrine inhibitor of sugar and alcohol appetite.
von Holstein-Rathlou S, Gillum MP. von Holstein-Rathlou S, et al. J Physiol. 2019 Jul;597(14):3539-3548. doi: 10.1113/JP277117. Epub 2019 Apr 14. J Physiol. 2019. PMID: 30921473 Review. - Nutritional regulation of fibroblast growth factor 21: from macronutrients to bioactive dietary compounds.
Pérez-Martí A, Sandoval V, Marrero PF, Haro D, Relat J. Pérez-Martí A, et al. Horm Mol Biol Clin Investig. 2016 Sep 1;30(1):/j/hmbci.2017.30.issue-1/hmbci-2016-0034/hmbci-2016-0034.xml. doi: 10.1515/hmbci-2016-0034. Horm Mol Biol Clin Investig. 2016. PMID: 27583468 Review.
Cited by
- A Dozen Years of Discovery: Insights into the Physiology and Pharmacology of FGF21.
Kliewer SA, Mangelsdorf DJ. Kliewer SA, et al. Cell Metab. 2019 Feb 5;29(2):246-253. doi: 10.1016/j.cmet.2019.01.004. Cell Metab. 2019. PMID: 30726758 Free PMC article. Review. - Nutritional Ketosis as a Potential Treatment for Alcohol Use Disorder.
Mahajan VR, Elvig SK, Vendruscolo LF, Koob GF, Darcey VL, King MT, Kranzler HR, Volkow ND, Wiers CE. Mahajan VR, et al. Front Psychiatry. 2021 Nov 30;12:781668. doi: 10.3389/fpsyt.2021.781668. eCollection 2021. Front Psychiatry. 2021. PMID: 34916977 Free PMC article. Review. - FGF 21 deficiency slows gastric emptying and reduces initial blood alcohol concentration in mice exposed to acute alcohol in fasting state.
Wu G, Liu Y, Liu Y, Zhang L, Zhao H, Liu L, Zhao C, Feng W. Wu G, et al. Biochem Biophys Res Commun. 2018 Feb 26;497(1):46-50. doi: 10.1016/j.bbrc.2018.01.189. Epub 2018 Feb 12. Biochem Biophys Res Commun. 2018. PMID: 29448103 Free PMC article. - The role of ChREBP in carbohydrate sensing and NAFLD development.
Régnier M, Carbinatti T, Parlati L, Benhamed F, Postic C. Régnier M, et al. Nat Rev Endocrinol. 2023 Jun;19(6):336-349. doi: 10.1038/s41574-023-00809-4. Epub 2023 Apr 13. Nat Rev Endocrinol. 2023. PMID: 37055547 Review. - ChREBP refines the hepatic response to fructose to protect the liver from injury.
Hall AM, Finck BN. Hall AM, et al. J Clin Invest. 2017 Jun 30;127(7):2533-2535. doi: 10.1172/JCI95008. Epub 2017 Jun 19. J Clin Invest. 2017. PMID: 28628039 Free PMC article.
References
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5P50 CA70907-16/CA/NCI NIH HHS/United States
- R01CA152301/CA/NCI NIH HHS/United States
- R01 CA152301/CA/NCI NIH HHS/United States
- R01 DK067158/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P50 CA070907/CA/NCI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01DK067158/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases