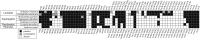Detecting and Characterizing the Highly Divergent Plastid Genome of the Nonphotosynthetic Parasitic Plant Hydnora visseri (Hydnoraceae) - PubMed (original) (raw)
. 2016 Jan 6;8(2):345-63.
doi: 10.1093/gbe/evv256.
Joshua P Der 2, Eric K Wafula 3, Samuel S Jones 4, Sarah T Wagner 5, Loren A Honaas 3, Paula E Ralph 3, Jay F Bolin 6, Erika Maass 7, Christoph Neinhuis 5, Stefan Wanke 5, Claude W dePamphilis 4
Affiliations
- PMID: 26739167
- PMCID: PMC4779604
- DOI: 10.1093/gbe/evv256
Detecting and Characterizing the Highly Divergent Plastid Genome of the Nonphotosynthetic Parasitic Plant Hydnora visseri (Hydnoraceae)
Julia Naumann et al. Genome Biol Evol. 2016.
Abstract
Plastid genomes of photosynthetic flowering plants are usually highly conserved in both structure and gene content. However, the plastomes of parasitic and mycoheterotrophic plants may be released from selective constraint due to the reduction or loss of photosynthetic ability. Here we present the greatly reduced and highly divergent, yet functional, plastome of the nonphotosynthetic holoparasite Hydnora visseri (Hydnoraceae, Piperales). The plastome is 27 kb in length, with 24 genes encoding ribosomal proteins, ribosomal RNAs, tRNAs, and a few nonbioenergetic genes, but no genes related to photosynthesis. The inverted repeat and the small single copy region are only approximately 1.5 kb, and intergenic regions have been drastically reduced. Despite extreme reduction, gene order and orientation are highly similar to the plastome of Piper cenocladum, a related photosynthetic plant in Piperales. Gene sequences in Hydnora are highly divergent and several complementary approaches using the highest possible sensitivity were required for identification and annotation of this plastome. Active transcription is detected for all of the protein-coding genes in the plastid genome, and one of two introns is appropriately spliced out of rps12 transcripts. The whole-genome shotgun read depth is 1,400× coverage for the plastome, whereas the mitochondrial genome is covered at 40× and the nuclear genome at 2×. Despite the extreme reduction of the genome and high sequence divergence, the presence of syntenic, long transcriptionally active open-reading frames with distant similarity to other plastid genomes and a high plastome stoichiometry relative to the mitochondrial and nuclear genomes suggests that the plastome remains functional in H. visseri. A four-stage model of gene reduction, including the potential for complete plastome loss, is proposed to account for the range of plastid genomes in nonphotosynthetic plants.
Keywords: Hydnoraceae; holoparasite; nonphotosynthetic; parasitic plants; plastid genome; plastome.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
Fig. 1.—
Hydnora visseri (A). Flower (B). Excavated underground stem (dark) connected to host plant Euphorbia gregaria (light) and a close-up. (C) Flower bud of H. visseri in foreground next to shovel and E. gregaria stems in background (D). Cross section of H. visseri underground stem.
Fig. 2.—
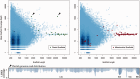
Plot of read depths relative to scaffold length. Cyan circles are all scaffolds from the genomic assembly creating darker spots at greater read depths. Scaffolds containing BLAST hits to plastid genes are overplotted by green filled circles (left). Scaffolds containing BLAST hits to mitochondrial genes are overplotted by red filled circles (right). All scaffolds at around 40× coverage that contain plastid gene fragments also contain mitochondrial genes. This indicates plastid sequences that have migrated into the mitochondrial genome. The two green filled circles at around 1,400× coverage (filled black arrows) contain only plastid genes and comprise the Hydnora plastid genome. The remaining cyan circles correspond to the nuclear genome. The remaining red and green filled circles are presumably mitochondrial and plastid sequences that are located in the nucleus.
Fig. 3.—
Light microscopy of two different starch-containing tissues of Hydnora visseri. (A) Section of tepal, starch grains in cells stained with iodine–potassium iodide. (B) Section of tepal, showing starch grains under polarized light; inset: enlarged starch grains. (C) Transverse section of underground organ; stained with Astrablue-safranin; 5-merous organization of vascular system is visible. (D) Starch grains in the underground organ; stained with iodine-potassium iodide. (E) Starch grains and vascular bundle in underground organ under polarized light.
Fig. 4.—
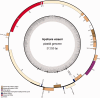
Map of the plastid genome of Hydnora visseri. Genes are color coded according to the legend, which indicates functional groups. The inner ring illustrates the boundaries of the LSC and SSC regions, separated by the two copies of the inverted repeat (IRa, IRb). The innermost ring shows the GC content across the plastid genome.
Fig. 5.—
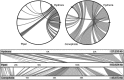
Maps of plastid genomes showing colinearity. The two circles show annotation-based syntenic regions between Hydnora and Piper and, respectively, Hydnora and Conopholis (generated in mGSV,
http://cas-bioinfo.cas.unt.edu/mgsv/
, last accessed January 11, 2016). At the bottom, there is a comparison between all three species. Gene order is colinear with the exception of an inversion of a large gene block between Hydnora and Conopholis that originally stems from opposite IRs.
Fig. 6.—
Gene content of highly reduced plastid genomes of seven nonphotosynthetic plants. The gene set shown here excludes gene categories that are missing in most of these extremely reduced plastomes. Black boxes indicate a present gene, white boxes indicate absent gene, and gray boxes indicate a pseudogene. A more comprehensive version of this figure including the full plastome gene set and including the available complete plastomes of hemiparasitic or nonphotosynthetic plants, as well as close photosynthetic relatives, is shown in
supplementary figure S4
,
Supplementary Material
online.
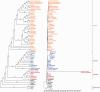
Fig. 7.—
ML phylogenetic trees of the Hydnora visseri plastid genes. A phylogenetic tree has been estimated for a concatenated data set of 20 plastid genes, based on alignments published by Jansen et al. (2007). The tree was estimated with RAxML (Stamatakis 2006), applying the rapid bootstrapping algorithm (1,000 bootstrap replicates). The topology of Jansen et al. (2007) was used as a starting tree (-t function in RAxML). The cladogram (left) verifies a magnoliid origin of the Hydnora plastid genes. Bootstrap values are plotted above nodes. The scale of the phylogram is in substitutions per site. The phylogram (right) shows a much higher number of substitutions for the Hydnora sequences compared with the other taxa.
Similar articles
- The loss of photosynthetic pathways in the plastid and nuclear genomes of the non-photosynthetic mycoheterotrophic eudicot Monotropa hypopitys.
Ravin NV, Gruzdev EV, Beletsky AV, Mazur AM, Prokhortchouk EB, Filyushin MA, Kochieva EZ, Kadnikov VV, Mardanov AV, Skryabin KG. Ravin NV, et al. BMC Plant Biol. 2016 Nov 16;16(Suppl 3):238. doi: 10.1186/s12870-016-0929-7. BMC Plant Biol. 2016. PMID: 28105941 Free PMC article. - The First Plastid Genome of the Holoparasitic Genus Prosopanche (Hydnoraceae).
Jost M, Naumann J, Rocamundi N, Cocucci AA, Wanke S. Jost M, et al. Plants (Basel). 2020 Mar 1;9(3):306. doi: 10.3390/plants9030306. Plants (Basel). 2020. PMID: 32121567 Free PMC article. - Comprehensive genomic analyses with 115 plastomes from algae to seed plants: structure, gene contents, GC contents, and introns.
Kwon EC, Kim JH, Kim NS. Kwon EC, et al. Genes Genomics. 2020 May;42(5):553-570. doi: 10.1007/s13258-020-00923-x. Epub 2020 Mar 21. Genes Genomics. 2020. PMID: 32200544 - The ins and outs of editing and splicing of plastid RNAs: lessons from parasitic plants.
Tillich M, Krause K. Tillich M, et al. N Biotechnol. 2010 Jul 31;27(3):256-66. doi: 10.1016/j.nbt.2010.02.020. Epub 2010 Mar 3. N Biotechnol. 2010. PMID: 20206308 Review. - From chloroplasts to "cryptic" plastids: evolution of plastid genomes in parasitic plants.
Krause K. Krause K. Curr Genet. 2008 Sep;54(3):111-21. doi: 10.1007/s00294-008-0208-8. Epub 2008 Aug 12. Curr Genet. 2008. PMID: 18696071 Review.
Cited by
- Understanding the evolution of holoparasitic plants: the complete plastid genome of the holoparasite Cytinus hypocistis (Cytinaceae).
Roquet C, Coissac É, Cruaud C, Boleda M, Boyer F, Alberti A, Gielly L, Taberlet P, Thuiller W, Van Es J, Lavergne S. Roquet C, et al. Ann Bot. 2016 Oct 1;118(5):885-896. doi: 10.1093/aob/mcw135. Ann Bot. 2016. PMID: 27443299 Free PMC article. - Genomic comparison of non-photosynthetic plants from the family Balanophoraceae with their photosynthetic relatives.
Schelkunov MI, Nuraliev MS, Logacheva MD. Schelkunov MI, et al. PeerJ. 2021 Aug 31;9:e12106. doi: 10.7717/peerj.12106. eCollection 2021. PeerJ. 2021. PMID: 34540375 Free PMC article. - Transit From Autotrophism to Heterotrophism: Sequence Variation and Evolution of Chloroplast Genomes in Orobanchaceae Species.
Zhang R, Xu B, Li J, Zhao Z, Han J, Lei Y, Yang Q, Peng F, Liu ZL. Zhang R, et al. Front Genet. 2020 Oct 6;11:542017. doi: 10.3389/fgene.2020.542017. eCollection 2020. Front Genet. 2020. PMID: 33133143 Free PMC article. - The Complete Plastomes of Five Hemiparasitic Plants (Osyris wightiana, Pyrularia edulis, Santalum album, Viscum liquidambaricolum, and V. ovalifolium): Comparative and Evolutionary Analyses Within Santalales.
Guo X, Liu C, Zhang G, Su W, Landis JB, Zhang X, Wang H, Ji Y. Guo X, et al. Front Genet. 2020 Jun 16;11:597. doi: 10.3389/fgene.2020.00597. eCollection 2020. Front Genet. 2020. PMID: 32612639 Free PMC article. - Evolutionary differences in gene loss and pseudogenization among mycoheterotrophic orchids in the tribe Vanilleae (subfamily Vanilloideae).
Zhou L, Chen T, Qiu X, Liu J, Guo S. Zhou L, et al. Front Plant Sci. 2023 Mar 22;14:1160446. doi: 10.3389/fpls.2023.1160446. eCollection 2023. Front Plant Sci. 2023. PMID: 37035052 Free PMC article.
References
- Adam Z, Clarke AK. 2002. Cutting edge of chloroplast proteolysis. Trends Plant Sci. 7(10):451–456. - PubMed
- Baldauf SL, Palmer JD. 1990. Evolutionary transfer of the chloroplast tufA gene to the nucleus. Nature 344:262–265. - PubMed
- Barbrook AC, Howe CJ, Purton S. 2006. Why are plastid genomes retained in non-photosynthetic organisms? Trends Plant Sci.. 11:101–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous