Maternal immune activation produces neonatal excitability defects in offspring hippocampal neurons from pregnant rats treated with poly I:C - PubMed (original) (raw)
Maternal immune activation produces neonatal excitability defects in offspring hippocampal neurons from pregnant rats treated with poly I:C
Eti Patrich et al. Sci Rep. 2016.
Abstract
Maternal immune activation (MIA) resulting from prenatal exposure to infectious pathogens or inflammatory stimuli is increasingly recognized to play an important etiological role in neuropsychiatric disorders with neurodevelopmental features. MIA in pregnant rodents induced by injection of the synthetic double-stranded RNA, Poly I:C, a mimic of viral infection, leads to a wide spectrum of behavioral abnormalities as well as structural and functional defects in the brain. Previous MIA studies using poly I:C prenatal treatment suggested that neurophysiological alterations occur in the hippocampus. However, these investigations used only juvenile or adult animals. We postulated that MIA-induced alterations could occur earlier at neonatal/early postnatal stages. Here we examined the neurophysiological properties of cultured pyramidal-like hippocampal neurons prepared from neonatal (P0-P2) offspring of pregnant rats injected with poly I:C. Offspring neurons from poly I:C-treated mothers exhibited significantly lower intrinsic excitability and stronger spike frequency adaptation, compared to saline. A similar lower intrinsic excitability was observed in CA1 pyramidal neurons from hippocampal slices of two weeks-old poly I:C offspring. Cultured hippocampal neurons also displayed lower frequency of spontaneous firing, higher charge transfer of IPSCs and larger amplitude of miniature IPSCs. Thus, maternal immune activation leads to strikingly early neurophysiological abnormalities in hippocampal neurons.
Figures
Figure 1. Cultured hippocampal neurons from offspring of polyI:C-injected mothers exhibit lower intrinsic excitability properties.
(A) Representative superimposed traces of single action potentials from cultured hippocampal neurons from offspring of saline (left panel) and polyI:C (right panel) -injected mothers. Solitary spike was evoked by 2 ms squared pulse injection of current steps starting from 200 pA in 50 pA increments, in the presence of glutamate and GABAA ionotropic receptors blockers (10 μM NBQX, 10 μM AP-5, 30 μM picrotoxin and 10 μM bicuculline methyl iodide). The line represents the starting resting potential before current injection. (B) Significantly higher current injection was required to evoke a single spike in cultured hippocampal neurons from offspring of polyI:C-injected mothers compared to those from offspring of saline-injected mothers (594 ± 25 pA, n = 54 from 6 poly I:C-injected mothers versus 409 ± 19 pA, n = 44 from 6 saline-injected mothers, t(96) = 5.69, p < 0.0001). (C) Representative traces of evoked spiking activity of cultured hippocampal neurons from offspring of saline (left panel) and polyI:C (right panel) -injected mothers. Spiking activity was evoked by 800 ms squared pulse injection of series of current steps starting from −300 pA up to 250 pA in 50 pA increments, in the presence of the glutamate and GABAA ionotropic receptors blockers. (D) Cultured hippocampal neurons from offspring of polyI:C-injected mothers exhibited significantly lower membrane input resistance compared to those of saline-injected mothers (Rin = 200 ± 8 MΩ, n = 56 from 6 polyI:C-injected mothers versus Rin = 236 ± 9 MΩ, n = 41 from 6 saline-injected mothers; t(95) = −2.79, p < 0.01). (E) frequency-current plots showing that the firing frequency in cultured hippocampal neurons from offspring of polyI:C-injected mothers is lower compared to that from offspring of saline-injected mothers. Repeated ANOVA yielded main effects of current injection, prenatal treatment and significant interaction between current injection and prenatal treatment (F(5, 450) = 235.2, p < 0.0001; F(1, 90) = 11.81, p < 0.001 and F(5, 450) = 3.7, P < 0.003 respectively; post hoc p’s < 0.05; n = 55 from 6 polyI:C-injected mothers versus n = 37 from offspring of 6 saline-injected mothers).
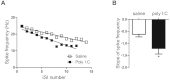
Figure 2. Cultured hippocampal neurons from offspring of polyI:C-injected mothers exhibit stronger spike frequency adaptation.
(A) Representative frequency-interspike interval (ISI) plot showing the stronger spike frequency adaptation as calculated by the slope of the linear regression of the spike frequency as a function of the ISI number. (B) Hippocampal neurons from offspring of polyI:C-injected mothers exhibit a larger slope (S) of spike frequency adaptation than those from offspring of saline-injected moms (S = −1.21 ± 0.22, n = 35 from 6 polyI:C-injected mothers versus S = −0.62 ± 0.11, n = 38 from 6 saline-injected mothers; t(71) = −2.25, p < 0.05).
Figure 3. CA1 pyramidal neurons of acute hippocampal slices from juvenile offspring of polyI:C-injected mothers exhibit lower intrinsic excitability.
(A) Representative superimposed traces of single action potentials from cultured hippocampal neurons from offspring of saline (left panel) and polyI:C (right panel) -injected mothers. Solitary spike was evoked by 2 ms squared pulse injection of current steps starting from 50 pA in 50 pA increments. (B) CA1 pyramidal neurons from offspring of polyI:C-injected mothers have significantly higher rheobase current compared to those from offspring of saline-injected mothers (398 ± 36 pA, n = 24 from 7 polyI:C-injected mothers versus 257 ± 22 pA, n = 27 from 6 saline-injected mothers, t(49) = 3.34, p < 0.001). (C) representative traces of evoked spiking activity of CA1 pyramidal neurons from offspring of saline (top)- and polyI:C (lower)-injected mothers, respectively. Spiking activity was evoked by 400 ms squared pulse injection of series of current steps starting from 0 pA up to 225 pA in 25 pA increments. (D) The spike frequency of offspring from polyI:C-injected mothers is lower compared to that from saline-injected mothers. Repeated ANOVA yielded main effects of stimulation current, prenatal treatment and significant interaction between stimulation current and prenatal treatment (F(9, 369) = 272.8, p < 0.0001; F(1, 41) = 10.86, p < 0.005; F(9, 369) = 2.65, p < 0.006, respectively; post hoc p’s < 0.05; n = 21 from offspring of 6 polyI:C injected mothers versus, n = 22 from offspring of 6 saline injected mothers).
Figure 4. Cultured hippocampal neurons from offspring of polyI:C injected mothers exhibit lower frequency of spontaneous spike discharge.
(A) Representative current-clamp recording of ongoing spiking activity of cultured hippocampal neurons from offspring of saline (top) and poly I:C (bottom) -injected mothers. (B) Cultured hippocampal neurons of offspring from polyI:C-injected mothers display significantly lower frequency of spontaneous spike discharge compared to that of offspring from saline-injected mothers (F = 1.30 ± 0.20, n = 57 from 6 polyI:C-injected mothers versus F = 2.21 ± 0.27, n = 46 from 6 saline-injected mothers; t(101) = −2.77, p < 0.01).
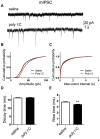
Figure 5. Cultured hippocampal neurons from offspring of polyI:C-treated mothers exhibit no change in sEPSC charge transfer but larger charge transfer of sIPSC.
(A) Representative voltage-clamp recording of sEPSC of cultured hippocampal neurons from offspring of saline (left) and polyI:C (right)-injected mothers. (B) Cultured hippocampal neurons from the offspring of polyI:C treated mothers display similar sEPSC charge transfer compared to that of offspring from saline-treated mothers (Q = 99 ± 10 pA*s, n = 44 from 6 polyI:C-injected mothers Q = 103 ± 10 pA*s, n = 41 from 6 saline-injected mothers). (C) Representative voltage-clamp recording of sIPSC of cultured hippocampal neurons from offspring of saline (left) and polyI:C (right)-injected mothers. (D) Cultured hippocampal neurons from offspring of polyI:C-treated mothers exhibit larger sIPSC charge transfer compared to those from offspring of saline-treated mothers (Q = 173 ± 23 pA*s, n = 38 from 6 polyI:C-injected mothers versus Q = 112 ± 16 pA*s, n = 47 from 6 saline-injected mothers; t(83) = 2.25, p < 0.03).
Figure 6. Cultured hippocampal neurons from offspring of saline and polyI:C-treated mothers exhibit similar mEPSC properties.
(A) Representative voltage-clamp recording of mEPSCs in cultured hippocampal neurons from offspring of saline (top) and polyI:C (bottom)-injected mothers. (B) Cumulative probability-amplitude plots show no differences in the mEPSC amplitude of cultured hippocampal neurons from offspring of saline and polyI:C-injected mothers (n = 35 cells and n = 40 cells, respectively, in each group from 6 different mothers). (C) Cumulative probability-interevent interval plots show no differences in the mEPSC frequency of cultured hippocampal neurons from offspring of saline and polyI:C-injected mothers (n = 32 cells and n = 37 cells, respectively, in each group from 6 different mothers). (D) No differences were found in the mEPSC decay time in cultured hippocampal neurons from offspring of saline and polyI:C-injected mothers (n = 35 cells and n = 40 cells, respectively, in each group from 6 different mothers). (E) No differences were found in the mEPSC rise time in cultured hippocampal neurons from offspring of saline and polyI:C-injected mothers (n = 35 cells and n = 40 cells, respectively, in each group from 6 different mothers). For each recorded neuron, 200 events were analyzed.
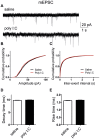
Figure 7. Cultured hippocampal neurons from offspring of polyI:C injected mothers exhibit larger amplitude and shorter rise time of mIPSCs.
(A) Representative voltage-clamp recording of mIPSCs in cultured hippocampal neurons from offspring of saline (top) and polyI:C (bottom)-injected mothers. (B) Cumulative probability-amplitude plots show larger amplitude of mIPSCs in cultured hippocampal neurons from offspring and polyI:C-injected mothers compared to that from offspring of saline-treated moms (I = 43.4 ± 0.5 pA, n = 28 cells from 6 polyI:C-injected mothers versus I = 38.6 ± 0.5 pA, n = 30 cells from 6 saline-injected mothers; p < 0.0001). (C) Cumulative probability-interevent interval plots show no differences in the mIPSC frequency of cultured hippocampal neurons from offspring of saline and polyI:C-injected mothers (n = 27 cells and n = 24 cells, respectively, in each group from 6 different mothers). (D) no differences were found in the mIPSC decay time in cultured hippocampal neurons from offspring of saline and polyI:C-injected mothers (n = 30 cells and n = 28 cells, respectively, in each group from 6 different mothers). (E) Cultured hippocampal neurons from offspring of polyI:C injected mothers exhibit shorter mean rise time of mIPSCs (3.4 ± 0.06 ms, n = 28 cells from 6 polyI:C-injected mothers versus 3.8 ± 0.06 ms ,n = 30 cells from 6 saline-injected mothers; t(2733) = −4.88, p < 0.0001). For each recorded neuron, 50 events were analyzed.
Similar articles
- Prenatal immune activation alters hippocampal place cell firing characteristics in adult animals.
Wolff AR, Bilkey DK. Wolff AR, et al. Brain Behav Immun. 2015 Aug;48:232-43. doi: 10.1016/j.bbi.2015.03.012. Epub 2015 Apr 3. Brain Behav Immun. 2015. PMID: 25843370 - Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring.
Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. Oh-Nishi A, et al. Brain Res. 2010 Dec 2;1363:170-9. doi: 10.1016/j.brainres.2010.09.054. Epub 2010 Sep 21. Brain Res. 2010. PMID: 20863817 - Tracing the development of psychosis and its prevention: what can be learned from animal models.
Piontkewitz Y, Arad M, Weiner I. Piontkewitz Y, et al. Neuropharmacology. 2012 Mar;62(3):1273-89. doi: 10.1016/j.neuropharm.2011.04.019. Epub 2011 Jun 23. Neuropharmacology. 2012. PMID: 21703648 Review. - Controversies and prospects about microglia in maternal immune activation models for neurodevelopmental disorders.
Smolders S, Notter T, Smolders SMT, Rigo JM, Brône B. Smolders S, et al. Brain Behav Immun. 2018 Oct;73:51-65. doi: 10.1016/j.bbi.2018.06.001. Epub 2018 Jun 2. Brain Behav Immun. 2018. PMID: 29870753 Review.
Cited by
- Maternal immune activation generates anxiety in offspring: A translational meta-analysis.
Quagliato LA, de Matos U, Nardi AE. Quagliato LA, et al. Transl Psychiatry. 2021 Apr 26;11(1):245. doi: 10.1038/s41398-021-01361-3. Transl Psychiatry. 2021. PMID: 33903587 Free PMC article. - Maternal stressors and the developmental origins of neuropsychiatric risk.
Khambadkone SG, Cordner ZA, Tamashiro KLK. Khambadkone SG, et al. Front Neuroendocrinol. 2020 Apr;57:100834. doi: 10.1016/j.yfrne.2020.100834. Epub 2020 Feb 18. Front Neuroendocrinol. 2020. PMID: 32084515 Free PMC article. Review. - Maternal immune activation alters glutamic acid decarboxylase-67 expression in the brains of adult rat offspring.
Cassella SN, Hemmerle AM, Lundgren KH, Kyser TL, Ahlbrand R, Bronson SL, Richtand NM, Seroogy KB. Cassella SN, et al. Schizophr Res. 2016 Mar;171(1-3):195-9. doi: 10.1016/j.schres.2016.01.041. Epub 2016 Jan 29. Schizophr Res. 2016. PMID: 26830319 Free PMC article. - Maternal Immune Activation Affects Hippocampal Excitatory and Inhibitory Synaptic Transmission in Offspring From an Early Developmental Period to Adulthood.
Nakagawa K, Yoshino H, Ogawa Y, Yamamuro K, Kimoto S, Noriyama Y, Makinodan M, Yamashita M, Saito Y, Kishimoto T. Nakagawa K, et al. Front Cell Neurosci. 2020 Aug 4;14:241. doi: 10.3389/fncel.2020.00241. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32903758 Free PMC article. - Compromised Hippocampal Neuroplasticity in the Interferon-α and Toll-like Receptor-3 Activation-Induced Mouse Depression Model.
Sanchez-Mendoza EH, Camblor-Perujo S, Martins Nascentes-Melo L, Dzyubenko E, Fleischer M, Silva de Carvalho T, Schmitt LI, Leo M, Hagenacker T, Herring A, Keyvani K, Bera S, Kononenko N, Kleinschnitz C, Hermann DM. Sanchez-Mendoza EH, et al. Mol Neurobiol. 2020 Jul;57(7):3171-3182. doi: 10.1007/s12035-020-01927-0. Epub 2020 Jun 5. Mol Neurobiol. 2020. PMID: 32504419 Free PMC article.
References
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 24, 881–897 (2010). - PubMed
- Brown A. S., Cohen P., Greenwald S. & Susser E. Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry 157, 438–443 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous