After Intracerebral Hemorrhage, Oligodendrocyte Precursors Proliferate and Differentiate Inside White-Matter Tracts in the Rat Striatum - PubMed (original) (raw)
After Intracerebral Hemorrhage, Oligodendrocyte Precursors Proliferate and Differentiate Inside White-Matter Tracts in the Rat Striatum
Michael J E Joseph et al. Transl Stroke Res. 2016 Jun.
Abstract
Damage to myelinated axons contributes to neurological deficits after acute CNS injury, including ischemic and hemorrhagic stroke. Potential treatments to promote re-myelination will require fully differentiated oligodendrocytes, but almost nothing is known about their fate following intracerebral hemorrhage (ICH). Using a rat model of ICH in the striatum, we quantified survival, proliferation, and differentiation of oligodendrocyte precursor cells (OPCs) (at 1, 3, 7, 14, and 28 days) in the peri-hematoma region, surrounding striatum, and contralateral striatum. In the peri-hematoma, the density of Olig2(+) cells increased dramatically over the first 7 days, and this coincided with disorganization and fragmentation of myelinated axon bundles. Very little proliferation (Ki67(+)) of Olig2(+) cells was seen in the anterior subventricular zone from 1 to 28 days. However, by 3 days, many were proliferating in the peri-hematoma region, suggesting that local proliferation expands their population. By 14 days, the density of Olig2(+) cells declined in the peri-hematoma region, and, by 28 days, it reached the low level seen in the contralateral striatum. At these later times, many surviving axons were aligned into white-matter bundles, which appeared less swollen or fragmented. Oligodendrocyte cell maturation was prevalent over the 28-day period. Densities of immature OPCs (NG2(+)Olig2(+)) and mature (CC-1(+)Olig2(+)) oligodendrocytes in the peri-hematoma increased dramatically over the first week. Regardless of the maturation state, they increased preferentially inside the white-matter bundles. These results provide evidence that endogenous oligodendrocyte precursors proliferate and differentiate in the peri-hematoma region and have the potential to re-myelinate axon tracts after hemorrhagic stroke.
Keywords: Corticostriatal axons; Hemorrhagic stroke; Myelinated axon tracts; Oligodendrocyte maturation; Peri-hematoma recovery; Post-stroke recovery.
Figures
Fig. 1
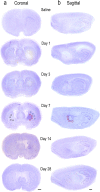
Hematoma development in the first month after ICH. Representative cresyl violet-stained sections from rats on day 1 after saline injection (control) and on days 1, 3, 7, 14, and 28 after injection of type IV collagenase into the right striatum. For day 7 after ICH, sample boxes are shown for the peri-hematoma (red), surrounding ipsilateral striatum (blue), and contralateral striatum (black). a Coronal sections. The hematoma appears pale, which is especially clear on the right side of the sections on days 1 and 3. On days 7 and 14, the hematoma is smaller and darkly stained. b Sagittal sections. Scale bars = 1 mm
Fig. 2
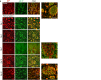
Myelin damage and axon survival in the peri-hematoma after ICH. Representative images of sections double-labeled with an antibody against neurofilaments (_pan_-NF; green) and an antibody against myelin basic protein (MBP; red). a Coronal sections from the region adjacent to the hematoma, which is to the right but beyond the image (as indicated by “→ H”). The peri-hematoma is visible in the _right_-most portion of each image, and the surrounding striatum is visible toward the left. Higher-magnification images of the boxed regions are shown to the right. b Sagittal sections. All images were taken in the peri-hematoma, the region immediately adjacent to the hematoma, which is below each portion shown (as indicated by “↓H”). Scale bars = 50 μm and apply to all panels
Fig. 3
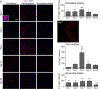
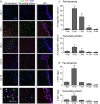
Numbers of oligodendrocyte-lineage cells increase after ICH, especially in the peri-hematoma. a Oligodendrocyte-lineage cells of any maturation state were identified using an antibody against the nuclear antigen, Olig2 (red). Cell nuclei were stained with DAPI (blue), and the enlarged inset shows an example of staining co-localization. Representative images from 1 to 28 days after ICH show the contralateral (undamaged) striatum, the peri-hematoma region, and the striatum surrounding the peri-hematoma. Scale bar = 50 μm (main panels), 10 μm (inset). Inset to right: Low-magnification image of oligodendrocyte-lineage cells (Olig2; red) surrounding the hematoma at 7 days. Scale bar = 50 μm. b Time-dependent changes in the density of oligodendrocyte-lineage cells were determined by counting double-labeled Olig2+DAPI+ nuclei. Values represent mean ± SEM from three rats at each time point, and the dashed line represents the mean for 1-day saline controls (n = 3). Statistical comparisons were based on a one-way ANOVA followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # between ICH and 1 day saline controls; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001, four symbols p < 0.0001. Additional significant differences that are not illustrated with symbols are as follows: (i) in the peri-hematoma, 7 vs 1 and 28 days; (ii) in the surrounding striatum, 28 vs 1, 3, and 7 days; and (iii) in the contralateral striatum, 28 vs 1, 3, and 7 days
Fig. 4
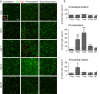
Oligodendrocyte-lineage cells preferentially increase inside white-matter bundles. a Representative images from sagittal sections taken from the peri-hematoma region, with myelin bundles labeled with MBP (green) and oligodendrocyte-lineage cells of any maturation state labeled with Olig2 (red). Scale bar = 50 μm and applies to all panels. b Density of Olig2+ cells inside or outside white-matter bundles in the peri-hematoma region. Values are mean ± SEM from four rats at each time point after ICH. The dashed lines indicate mean values for 1-day saline controls (n = 3) for oligodendrocyte-lineage cells inside (red) and outside bundles (black). Statistical comparisons were based on a two-way ANOVA followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the saline control, * between those inside and outside the bundles; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001
Fig. 5
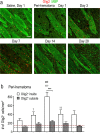
Oligodendrocyte-lineage cells proliferate in the damaged striatum, particularly in the peri-hematoma region. a Representative images show oligodendrocyte-lineage cells labeled for Olig2 (green), proliferating cells stained with an antibody against Ki67 (red), and nuclei labeled with DAPI (blue). Images were taken from the ipsilateral side in the peri-hematoma region (left panels), surrounding striatum (middle), and SVZ (right). Scale bar = 50 μm and applies to all panels. b Higher-magnification images of typical single-, double-, and triple-stained nuclei in each region at 3 days after ICH. c Density of proliferating oligodendrocyte-lineage cells (i.e., cells with Olig2+Ki67+ nuclei) in the peri-hematoma and surrounding striatum from 1 to 28 days after ICH. d Percentage of oligodendrocyte-lineage cells that were proliferating. Values are mean ± SEM from three rats at each time. Statistical comparisons were based on a one-way ANOVA followed by Tukey’s post-hoc test and show differences: † from the preceding time point; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001. Additional significant differences in the density of proliferating oligodendrocyte-lineage cells that are not indicated with symbols were as follows: (i) in the peri-hematoma, 1 vs 7 days, 3 vs 14 days, and 28 vs 3 and 7 days; and (ii) in the surrounding striatum, 3 vs 14 and 28 days. For the percentage of oligodendrocyte-lineage cells that were Ki67+, all time points were significantly different from 3 days in both the peri-hematoma and surrounding striatum
Fig. 6
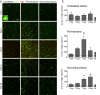
Oligodendrocyte precursor cells (OPCs) increase preferentially in the peri-hematoma and inside white-matter bundles. a OPCs were identified as cells with Olig2+ nuclei (red) that were double-labeled with an antibody against NG2 chondroitin sulfate proteoglycan (green). Representative images were taken from the contralateral striatum, and the peri-hematoma region and the surrounding striatum on the ipsilateral side. The higher-magnification inset shows a typical OPC, with an Olig2+ nucleus surrounded by NG2. Scale bar = 50 μm (main panels), 10 μm (inset). b Time-dependent changes in density of OPCs (Olig2+NG2+ cells). Values represent mean ± SEM from three rats at each time point, and the dashed line represents the mean for 1-day saline controls (n = 3). Statistical comparisons were based on a one-way ANOVA followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the 1-day saline control; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001, four symbols p < 0.0001. Additional significant differences that are not shown with symbols are as follows: (i) in the peri-hematoma, 7 vs 1 and 28 days, 3 vs 14 and 28 days; and (ii) in the surrounding striatum, 3 vs 14 and 28 days. c Representative images from sagittal sections taken from the peri-hematoma region were triple-stained for Olig2 (red), NG2 (green), and myelin basic protein (MBP; blue). Scale bar = 50 μm and applies to all panels. d Density of OPCs (Olig2+NG2+ cells) inside or outside white-matter bundles in the peri-hematoma region. Values are mean ± SEM from four rats at each time point after ICH. The dashed lines indicate mean levels for 1-day saline controls (n = 3) for OPCs inside (red) and outside the bundles (black). Statistical comparisons were based on a two-way ANOVA, followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the saline control, * between those inside and outside the bundles; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001
Fig. 6
Oligodendrocyte precursor cells (OPCs) increase preferentially in the peri-hematoma and inside white-matter bundles. a OPCs were identified as cells with Olig2+ nuclei (red) that were double-labeled with an antibody against NG2 chondroitin sulfate proteoglycan (green). Representative images were taken from the contralateral striatum, and the peri-hematoma region and the surrounding striatum on the ipsilateral side. The higher-magnification inset shows a typical OPC, with an Olig2+ nucleus surrounded by NG2. Scale bar = 50 μm (main panels), 10 μm (inset). b Time-dependent changes in density of OPCs (Olig2+NG2+ cells). Values represent mean ± SEM from three rats at each time point, and the dashed line represents the mean for 1-day saline controls (n = 3). Statistical comparisons were based on a one-way ANOVA followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the 1-day saline control; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001, four symbols p < 0.0001. Additional significant differences that are not shown with symbols are as follows: (i) in the peri-hematoma, 7 vs 1 and 28 days, 3 vs 14 and 28 days; and (ii) in the surrounding striatum, 3 vs 14 and 28 days. c Representative images from sagittal sections taken from the peri-hematoma region were triple-stained for Olig2 (red), NG2 (green), and myelin basic protein (MBP; blue). Scale bar = 50 μm and applies to all panels. d Density of OPCs (Olig2+NG2+ cells) inside or outside white-matter bundles in the peri-hematoma region. Values are mean ± SEM from four rats at each time point after ICH. The dashed lines indicate mean levels for 1-day saline controls (n = 3) for OPCs inside (red) and outside the bundles (black). Statistical comparisons were based on a two-way ANOVA, followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the saline control, * between those inside and outside the bundles; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001
Fig. 7
Mature oligodendrocytes increase after ICH, especially inside white-matter bundles in the peri-hematoma. a Mature oligodendrocytes were identified as cells with Olig2+ nuclei (red) that were double-labeled with an antibody against adenomatous polyposis coli (APC, also called CC-1; green). Representative images were taken from the contralateral striatum, and the peri-hematoma and surrounding striatum on the ipsilateral side. The higher-magnification inset shows a typical mature oligodendrocyte with an Olig2+ nucleus surrounded by CC-1. Scale bar = 50 μm (main panels), 10 μm (inset). b Time-dependent changes by density of mature oligodendrocytes (Olig2+CC-1+ cells). Values represent mean ± SEM from three rats at each time point, and the dashed line represents the mean for 1-day saline controls (n = 3). Statistical comparisons were based on a one-way ANOVA followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the 1-day saline control; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001. Additional significant differences that are not shown with symbols are as follows: (i) in the peri-hematoma, 7 vs 1 and 28 days; and (ii) in the surrounding striatum, 7 vs 1 and 28 days and 14 vs 1 and 3 days. c Representative images from sagittal sections from the peri-hematoma region that were triple-stained for Olig2 (red), CC-1 (green), and myelin basic protein (MBP; blue). Scale bar = 50 μm and applies to all panels. d Density of mature oligodendrocytes (Olig2+/CC-1+) inside or outside white-matter bundles in the peri-hematoma region. Values are mean ± SEM from four rats at each time point. The dashed lines indicate mean values for 1-day saline controls (n = 3) for oligodendrocytes inside (red) and outside the bundles (black). Statistical comparisons were based on a two-way ANOVA, followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the saline control, * between those inside and outside the bundles; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001
Fig. 7
Mature oligodendrocytes increase after ICH, especially inside white-matter bundles in the peri-hematoma. a Mature oligodendrocytes were identified as cells with Olig2+ nuclei (red) that were double-labeled with an antibody against adenomatous polyposis coli (APC, also called CC-1; green). Representative images were taken from the contralateral striatum, and the peri-hematoma and surrounding striatum on the ipsilateral side. The higher-magnification inset shows a typical mature oligodendrocyte with an Olig2+ nucleus surrounded by CC-1. Scale bar = 50 μm (main panels), 10 μm (inset). b Time-dependent changes by density of mature oligodendrocytes (Olig2+CC-1+ cells). Values represent mean ± SEM from three rats at each time point, and the dashed line represents the mean for 1-day saline controls (n = 3). Statistical comparisons were based on a one-way ANOVA followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the 1-day saline control; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001. Additional significant differences that are not shown with symbols are as follows: (i) in the peri-hematoma, 7 vs 1 and 28 days; and (ii) in the surrounding striatum, 7 vs 1 and 28 days and 14 vs 1 and 3 days. c Representative images from sagittal sections from the peri-hematoma region that were triple-stained for Olig2 (red), CC-1 (green), and myelin basic protein (MBP; blue). Scale bar = 50 μm and applies to all panels. d Density of mature oligodendrocytes (Olig2+/CC-1+) inside or outside white-matter bundles in the peri-hematoma region. Values are mean ± SEM from four rats at each time point. The dashed lines indicate mean values for 1-day saline controls (n = 3) for oligodendrocytes inside (red) and outside the bundles (black). Statistical comparisons were based on a two-way ANOVA, followed by Tukey’s post-hoc test. Comparisons show differences: † from the preceding time point, # from the saline control, * between those inside and outside the bundles; one symbol p < 0.05, two symbols p < 0.01, three symbols p < 0.001
Similar articles
- Region specific oligodendrocyte transcription factor expression in a model of neonatal hypoxic injury.
Affeldt BM, Obenaus A, Chan J, Pardo AC. Affeldt BM, et al. Int J Dev Neurosci. 2017 Oct;61:1-11. doi: 10.1016/j.ijdevneu.2017.05.001. Epub 2017 May 22. Int J Dev Neurosci. 2017. PMID: 28546087 - Diffuse traumatic brain injury in the mouse induces a transient proliferation of oligodendrocyte progenitor cells in injured white matter tracts.
Flygt J, Clausen F, Marklund N. Flygt J, et al. Restor Neurol Neurosci. 2017;35(2):251-263. doi: 10.3233/RNN-160675. Restor Neurol Neurosci. 2017. PMID: 27768001 - Chondroitin sulfate proteoglycans impede myelination by oligodendrocytes after perinatal white matter injury.
Deng YP, Sun Y, Hu L, Li ZH, Xu QM, Pei YL, Huang ZH, Yang ZG, Chen C. Deng YP, et al. Exp Neurol. 2015 Jul;269:213-23. doi: 10.1016/j.expneurol.2015.03.026. Epub 2015 Apr 8. Exp Neurol. 2015. PMID: 25862289 - Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury.
Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA. Armstrong RC, et al. Neuropharmacology. 2016 Nov;110(Pt B):654-659. doi: 10.1016/j.neuropharm.2015.04.029. Epub 2015 May 9. Neuropharmacology. 2016. PMID: 25963414 Review. - Subcortical ischemic vascular disease: Roles of oligodendrocyte function in experimental models of subcortical white-matter injury.
Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, Arai K. Shindo A, et al. J Cereb Blood Flow Metab. 2016 Jan;36(1):187-98. doi: 10.1038/jcbfm.2015.80. J Cereb Blood Flow Metab. 2016. PMID: 25920960 Free PMC article. Review.
Cited by
- Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury.
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G. Wang Z, et al. Transl Stroke Res. 2018 Feb;9(1):74-91. doi: 10.1007/s12975-017-0559-x. Epub 2017 Aug 1. Transl Stroke Res. 2018. PMID: 28766251 Free PMC article. - NLRP3 inflammasome and gut microbiota-brain axis: A new perspective on white matter injury after intracerebral hemorrhage.
Cai X, Cai X, Xie Q, Xiao X, Li T, Zhou T, Sun H. Cai X, et al. Neural Regen Res. 2026 Jan 1;21(1):62-80. doi: 10.4103/NRR.NRR-D-24-00917. Epub 2025 Jan 29. Neural Regen Res. 2026. PMID: 39885662 Free PMC article. - Intra-hematomal White Matter Tracts Act As a Scaffold for Macrophage Infiltration After Intracerebral Hemorrhage.
Chen J, Koduri S, Dai S, Toyota Y, Hua Y, Chaudhary N, Pandey AS, Keep RF, Xi G. Chen J, et al. Transl Stroke Res. 2021 Oct;12(5):858-865. doi: 10.1007/s12975-020-00870-5. Epub 2020 Oct 22. Transl Stroke Res. 2021. PMID: 33094829 Free PMC article. - White matter repair and treatment strategy after intracerebral hemorrhage.
Jiang YB, Wei KY, Zhang XY, Feng H, Hu R. Jiang YB, et al. CNS Neurosci Ther. 2019 Oct;25(10):1113-1125. doi: 10.1111/cns.13226. Epub 2019 Oct 2. CNS Neurosci Ther. 2019. PMID: 31578825 Free PMC article. Review. - P2X7 Participates in Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via MAPKs Signaling Pathways.
Wen Z, Mei B, Li H, Dou Y, Tian X, Shen M, Chen G. Wen Z, et al. Neurochem Res. 2017 Aug;42(8):2372-2383. doi: 10.1007/s11064-017-2257-1. Epub 2017 May 9. Neurochem Res. 2017. PMID: 28488233
References
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355–69. - PubMed
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167–76. - PubMed
- Poon MT, Fonville AF, Al-Shahi SR. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(6):660–7. - PubMed
- Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; J Cereb Circ. 2015;46(7):2032–60. - PubMed
- Anderson CS. Medical management of acute intracerebral hemorrhage. Curr Opin Crit Care. 2009;15(2):93–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources