Mechanical Characterization of a Dynamic and Tunable Methacrylated Hyaluronic Acid Hydrogel - PubMed (original) (raw)
Mechanical Characterization of a Dynamic and Tunable Methacrylated Hyaluronic Acid Hydrogel
Matthew G Ondeck et al. J Biomech Eng. 2016 Feb.
Abstract
Hyaluronic acid (HA) is a commonly used natural polymer for cell scaffolding. Modification by methacrylate allows it to be polymerized by free radicals via addition of an initiator, e.g., light-sensitive Irgacure, to form a methacrylated hyaluronic acid (MeHA) hydrogel. Light-activated crosslinking can be used to control the degree of polymerization, and sequential polymerization steps allow cells plated onto or in the hydrogel to initially feel a soft and then a stiff matrix. Here, the elastic modulus of MeHA hydrogels was systematically analyzed by atomic force microscopy (AFM) for a number of variables including duration of UV exposure, monomer concentration, and methacrylate functionalization. To determine how cells would respond to a specific two-step polymerization, NIH 3T3 fibroblasts were cultured on the stiffening MeHA hydrogels and found to reorganize their cytoskeleton and spread area upon hydrogel stiffening, consistent with cells originally cultured on substrates of the final elastic modulus.
Figures
Fig. 1
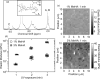
Functionalization and characterization of MeHA hydrogels. (a) NMR spectrum of 50 kDa HA with methacrylate functionalization (∼65% methacrylate functionalized). (b) Elastic modulus of 1% and 3% w/v of MeHA polymerized for 1, 2, and 3 min with 350 nm UV light. All samples are statistically different from one another based on one-way ANOVA with p < 10−4. (c) A 10 _μ_m × 10 _μ_m elastic modulus map for 1% and 3% MeHA gels UV polymerized for 1 min.
Fig. 2
Comparison of on-demand versus continuous stiffening. (a) Elastic modulus measured for 1% MeHA gels polymerized for 1, 2, and 4 min and gels polymerized for 1 and 2 min, stiffened additionally with 1 and 2 min of UV light exposure, respectively. Using nonparametric t-tests: *p < 10−12 and **p < 10−8. (b) Elastic modulus measured for 3% MeHA gels polymerized for 1, 2, and 4 min and gels polymerized for 1 and 2 min, stiffened additionally with 1 and 2 min of UV light exposure, respectively. Using nonparametric t-tests: *p < 10−7 and **p < 10−6. (c) A 10 _μ_m × 10 _μ_m elastic modulus map for 1% MeHA gel UV polymerized for 1 min then stiffened with an additional 1 min of UV light.
Fig. 3
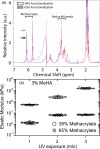
Impact of degree of methacrylation on stiffness. (a) NMR spectrum of MeHA with 38% (black arrow) and 65% functionalization (gray arrow), with peaks representing the methacrylate group and HA indicated. (b) Elastic modulus of 38% and 65% methacrylate functionalized 3% MeHA polymerized for 1, 2, and 3 min with 350 nm UV light. One-way ANOVA indicated that conditions were statistically different with p < 10−4 for UV exposure time within each methacrylation percentage, although post hoc Tukey analysis did not find a difference between 2 and 3 min exposure time for 1% MeHA.
Fig. 4
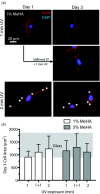
Dynamic stiffening affects cell spreading. (a) NIH 3T3 fibroblasts were cultured separately on 1% MeHA gels UV polymerized for 1 and 2 min, and dynamic MeHA gels polymerized for 1 min and then stiffened on for 1 additional minute. Cultures were stiffened on day 1 and fixed on day 3. Arrowheads indicate stress fibers. (b) Fibroblast cell area was measured at day 3 for cells cultured on 1% and 3% MeHA gels polymerized for 1 and 2 min and 1 + 1 min stiffened gels. The gray background is the range of cell areas for fibroblasts cultured on tissue culture glass as a comparison. One-way ANOVA indicated that only the 1% MeHA conditions were statistically different with p < 0.1 for UV exposure time.
Similar articles
- Preparation and Characterization of Photo-Cross-Linkable Methacrylated Silk Fibroin and Methacrylated Hyaluronic Acid Composite Hydrogels.
Amirian J, Wychowaniec JK, D Este M, Vernengo AJ, Metlova A, Sizovs A, Brangule A, Bandere D. Amirian J, et al. Biomacromolecules. 2024 Nov 11;25(11):7078-7097. doi: 10.1021/acs.biomac.4c00319. Epub 2024 Oct 14. Biomacromolecules. 2024. PMID: 39401165 Free PMC article. - Highly stretchable HA/SA hydrogels for tissue engineering.
Zhu C, Yang R, Hua X, Chen H, Xu J, Wu R, Cen L. Zhu C, et al. J Biomater Sci Polym Ed. 2018 Apr;29(5):543-561. doi: 10.1080/09205063.2018.1426425. Epub 2018 Jan 16. J Biomater Sci Polym Ed. 2018. PMID: 29316854 - Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair.
Fenn SL, Oldinski RA. Fenn SL, et al. J Biomed Mater Res B Appl Biomater. 2016 Aug;104(6):1229-36. doi: 10.1002/jbm.b.33476. Epub 2015 Jun 19. J Biomed Mater Res B Appl Biomater. 2016. PMID: 26097172 Free PMC article. - Hyaluronic acid hydrogels for biomedical applications.
Burdick JA, Prestwich GD. Burdick JA, et al. Adv Mater. 2011 Mar 25;23(12):H41-56. doi: 10.1002/adma.201003963. Epub 2011 Mar 10. Adv Mater. 2011. PMID: 21394792 Free PMC article. Review. - A Tunable Tumor Microenvironment through Recombinant Bacterial Collagen-Hyaluronic Acid Hydrogels.
Nemec S, Ganda S, Al Taief K, Kopecky C, Kuchel R, Lebhar H, Marquis CP, Thordarson P, Kilian KA. Nemec S, et al. ACS Appl Bio Mater. 2022 Jun 7. doi: 10.1021/acsabm.2c00186. Online ahead of print. ACS Appl Bio Mater. 2022. PMID: 35670558 Review.
Cited by
- Recent Advancements in Engineering Strategies for Manipulating Neural Stem Cell Behavior.
O'Grady BJ, Lippmann ES. O'Grady BJ, et al. Curr Tissue Microenviron Rep. 2020 Jun;1(2):41-47. doi: 10.1007/s43152-020-00003-y. Epub 2020 Apr 3. Curr Tissue Microenviron Rep. 2020. PMID: 33748772 Free PMC article. - Biphasic regulation of epigenetic state by matrix stiffness during cell reprogramming.
Song Y, Soto J, Wong SY, Wu Y, Hoffman T, Akhtar N, Norris S, Chu J, Park H, Kelkhoff DO, Ang CE, Wernig M, Kasko A, Downing TL, Poo MM, Li S. Song Y, et al. Sci Adv. 2024 Feb 16;10(7):eadk0639. doi: 10.1126/sciadv.adk0639. Epub 2024 Feb 14. Sci Adv. 2024. PMID: 38354231 Free PMC article. - Hydrogel biomaterials that stiffen and soften on demand reveal that skeletal muscle stem cells harbor a mechanical memory.
Madl CM, Wang YX, Holbrook CA, Su S, Shi X, Byfield FJ, Wicki G, Flaig IA, Blau HM. Madl CM, et al. Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2406787121. doi: 10.1073/pnas.2406787121. Epub 2024 Aug 20. Proc Natl Acad Sci U S A. 2024. PMID: 39163337 - Hyaluronic Acid-Based Hybrid Hydrogel Microspheres with Enhanced Structural Stability and High Injectability.
Seong YJ, Lin G, Kim BJ, Kim HE, Kim S, Jeong SH. Seong YJ, et al. ACS Omega. 2019 Aug 12;4(9):13834-13844. doi: 10.1021/acsomega.9b01475. eCollection 2019 Aug 27. ACS Omega. 2019. PMID: 31497700 Free PMC article. - Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling.
Ondeck MG, Kumar A, Placone JK, Plunkett CM, Matte BF, Wong KC, Fattet L, Yang J, Engler AJ. Ondeck MG, et al. Proc Natl Acad Sci U S A. 2019 Feb 26;116(9):3502-3507. doi: 10.1073/pnas.1814204116. Epub 2019 Feb 12. Proc Natl Acad Sci U S A. 2019. PMID: 30755531 Free PMC article.
References
- Paszek, M. J. , Zahir, N. , Johnson, K. R. , Lakins, J. N. , Rozenberg, G. I. , Gefen, A. , Reinhart-King, C. A. , Margulies, S. S. , Dembo, M. , Boettiger, D. , Hammer, D. A. , and Weaver, V. M. , 2005, “ Tensional Homeostasis and the Malignant Phenotype,” Cancer Cell, 8(3), pp. 241–254.10.1016/j.ccr.2005.08.010 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous