Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice - PubMed (original) (raw)
Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice
He Wu et al. J Cereb Blood Flow Metab. 2017 Jan.
Abstract
Inflammatory responses mediated by prostaglandins such as PGE2 may contribute to secondary brain injury after intracerebral hemorrhage (ICH). However, the cell-specific signaling by PGE2 receptor EP2 differs depending on whether the neuropathic insult is acute or chronic. Using genetic and pharmacologic approaches, we investigated the role of EP2 receptor in two mouse models of ICH induced by intrastriatal injection of collagenase or autologous arterial whole blood. We used middle-aged male mice to enhance the clinical relevance of the study. EP2 receptor was expressed in neurons but not in astrocytes or microglia after collagenase-induced ICH. Brain injury after collagenase-induced ICH was associated with enhanced cellular and molecular inflammatory responses, oxidative stress, and matrix metalloproteinase (MMP)-2/9 activity. EP2 receptor deletion exacerbated brain injury, brain swelling/edema, neuronal death, and neurobehavioral deficits, whereas EP2 receptor activation by the highly selective agonist AE1-259-01 reversed these outcomes. EP2 receptor deletion also exacerbated brain edema and neurologic deficits in the blood ICH model. These findings support the premise that neuronal EP2 receptor activation by PGE2 protects brain against ICH injury in middle-aged mice through its anti-inflammatory and anti-oxidant effects and anti-MMP-2/9 activity. PGE2/EP2 signaling warrants further investigation for potential use in ICH treatment.
Keywords: High-mobility group box 1; PGE2; inflammation; prostaglandin receptor.
© The Author(s) 2016.
Figures
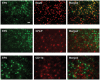
Figure 1.
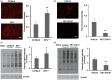
EP2 expression in the striatum of middle-aged mice subjected to collagenase-induced intracerebral hemorrhage (ICH). Double labeling immunofluorescence showed that EP2 receptor immunoreactivity (green) colocalized primarily with NeuN+ neurons (red), but not with GFAP+ astrocytes (red) or CD11b+ microglia/macrophages (red), in the peri-hematomal region on day 3 after collagenase-induced ICH. Three sections were analyzed per animal. Scale bar = 40 µm, n = 3 mice.
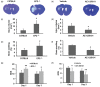
Figure 2.
Effect of EP2 receptor deletion or activation on brain lesion volume, brain swelling, and neurologic deficits in middle-aged male mice subjected to collagenase-induced intracerebral hemorrhage (ICH). (a, b) Representative Luxol fast blue/Cresyl Violet-stained brain sections on day 3 after ICH; injured areas lack staining. (c, e, g) Bar graphs show that EP2−/− mice had larger brain lesion volume (c), a greater degree of brain swelling (e), and more severe neurologic deficits (g) than did C57BL/6 wild-type mice. n = 8 mice/group. (d, f, h) Bar graphs show that mice treated with EP2 receptor agonist AE1-259-01 had smaller lesion volume (d), less brain swelling (f), and less severe neurologic deficits (h) than did mice treated with vehicle. Values are mean ± SD; n = 8 mice per group. *P < 0.05, **P < 0.01. NDS, neurologic deficit score.
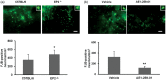
Figure 3.
Effect of EP2 receptor deletion or activation on neuronal death in middle-aged male mice subjected to collagenase-induced intracerebral hemorrhage (ICH). (a, c) Representative Fluoro-Jade B (FJB)-stained brain sections on day 3 after ICH showing intensely labeled neurons and processes in the perihematomal region. Scale bar = 30 µm. (b, d) Quantification analysis shows that EP2−/− mice had more FJB-positive neurons in the perihematomal region than did C57BL/6 wild-type mice (b), whereas mice treated with AE1-259-01 had fewer FJB-positive neurons in the perihematomal region than did vehicle-treated mice (d). Insets in (a, c) represent FJB-positive degenerating neurons at higher magnification. Values are means ± SD. n = 8 mice per group. *P < 0.05, **P < 0.01.
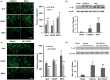
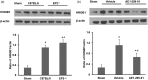
Figure 4.
Effects of EP2 receptor deletion or activation on cellular inflammatory responses and proinflammatory cytokine IL-1β production in middle-aged male mice subjected to collagenase-induced intracerebral hemorrhage (ICH). (a, b) In representative images, Iba1-, GFAP-, and MPO-immunopositive cells are evident around the hematoma on day 3 after ICH. Scale bar = 40 µm. Bar graphs show that EP2−/− mice had more activated microglia/macrophages, activated astrocytes, and infiltrating neutrophils than did C57BL/6 wild-type mice (a). n = 8 mice/group. In contrast, mice treated with AE1-259-01 had fewer activated microglia/macrophages, activated astrocytes, and infiltrating neutrophils than did mice treated with vehicle (b). n = 8 mice/group. (c, d) Western blot analysis shows that the level of IL-1β was increased in EP2−/− mice compared with that in C57BL/6 wild-type mice ((c), n = 5 mice/group) and was decreased in mice treated with AE1-259-01 compared with that in mice treated with vehicle ((d), n = 5 mice/group) on day 1 after ICH. β-actin was used as loading control. Values are means ± SD. *P < 0.05, **P < 0.01 versus C57BL/6 wild-type mice or vehicle-treated mice; #P < 0.05 versus sham mice.
Figure 5.
Effects of EP2 receptor deletion or activation on proinflammatory cytokine HMGB1 expression in middle-aged male mice subjected to collagenase-induced intracerebral hemorrhage (ICH). (a, b) Representative Western blots show the expression of HMGB1 protein on day 1 after ICH. Densitometric analysis shows that the level of HMGB1 protein increased in EP2−/− mice compared with that in C57BL/6 wild-type mice (a) and decreased in AE1-259-01-treated mice compared with that in vehicle-treated mice on day 1 after ICH. β-actin was used as a loading control. Values are means ± SD. n = 5 mice/group; *P < 0.05 versus C57BL/6 wild-type mice or vehicle-treated mice; #P < 0.05 versus sham mice.
Figure 6.
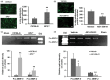
Effects of EP2 receptor deletion or activation on superoxide production and protein oxidation in middle-aged male mice subjected to collagenase-induced intracerebral hemorrhage (ICH). (a, b) In representative images, ethidium fluorescence (small red particles), a marker for superoxide production, was evident in the perihematomal region on day 1 after ICH. Scale bar: 30 µm. Bar graphs show the quantification analysis of ethidium fluorescence intensity in the perihematomal region. EP2−/− mice had greater signal intensity than did C57BL/6 wild-type mice (a), and mice treated with AE1-259-01 had less signal intensity than did mice treated with vehicle (b). (c, d) Representative immunoblots of hemorrhagic brain tissue. Bar graphs show that EP2−/− mice had a higher level of protein carbonylation than C57BL/6 wild-type mice (c) and that mice treated with AE1-259-01 had less protein carbonylation than mice treated with vehicle (d) on day 1 after ICH. Optical density was integrated over multiple protein bands for carbonyls. β-actin was used as loading control. Values are means ± SD. n = 5–8 mice/group; *P < 0.05, **P < 0.01.
Figure 7.
Effects of EP2 receptor deletion or activation on gelatinolytic activity in middle-aged male mice subjected to collagenase-induced intracerebral hemorrhage (ICH). (a, b) Representative gelatin in situ zymography fluorescent images of the perihematomal region from EP2−/− and C57BL/6 wild-type mice (a) and from vehicle- and AE1-259-01-treated mice (b) on day 1 after ICH. Scale bar: 30 µm. Bar graphs show that the number of gelatinolytic-positive cells was increased in EP2−/− mice compared with that in C57BL/6 wild-type mice (a) and decreased in AE1-259-01–treated mice compared with that in vehicle-treated mice (b). (c, d) Representative gelatin gel zymographs of MMP-2 and MMP-9 activity, visible as white bands on gels where gelatin was degraded. Bar graphs show that the gelatinolytic activity of pro-MMP-2 and pro-MMP-9 was greater in EP2−/− mice than in C57BL/6 wild-type mice (c) and less in AE1-259-01-treated mice than in vehicle-treated mice (d) on day 1 after ICH. Std, mouse gelatinase standards. Values are means ± SD. n = 5–8 mice/group; *P < 0.05, **P < 0.01.
Similar articles
- Prostaglandin E2 EP2 receptor deletion attenuates intracerebral hemorrhage-induced brain injury and improves functional recovery.
Leclerc JL, Lampert AS, Diller MA, Immergluck JB, Doré S. Leclerc JL, et al. ASN Neuro. 2015 Apr 13;7(2):1759091415578713. doi: 10.1177/1759091415578713. Print 2015 Mar-Apr. ASN Neuro. 2015. PMID: 25873308 Free PMC article. - Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice.
Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Li Q, Hou Z, Maruyama T, Zhang J, Wang J. Zhao X, et al. Brain Behav Immun. 2015 May;46:293-310. doi: 10.1016/j.bbi.2015.02.011. Epub 2015 Feb 16. Brain Behav Immun. 2015. PMID: 25697396 Free PMC article. - The Double Roles of the Prostaglandin E2 EP2 Receptor in Intracerebral Hemorrhage.
Luo X, Zhu Q, Zhang J, Huang Q, Xie Z, Cheng Y. Luo X, et al. Curr Drug Targets. 2017;18(12):1377-1385. doi: 10.2174/1389450117666151209122826. Curr Drug Targets. 2017. PMID: 26648067 Review. - Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase reduces brain damage and attenuates neuroinflammation after intracerebral hemorrhage.
Wu CH, Shyue SK, Hung TH, Wen S, Lin CC, Chang CF, Chen SF. Wu CH, et al. J Neuroinflammation. 2017 Nov 25;14(1):230. doi: 10.1186/s12974-017-1005-4. J Neuroinflammation. 2017. PMID: 29178914 Free PMC article. - Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection.
Jiang J, Dingledine R. Jiang J, et al. Trends Pharmacol Sci. 2013 Jul;34(7):413-23. doi: 10.1016/j.tips.2013.05.003. Epub 2013 Jun 21. Trends Pharmacol Sci. 2013. PMID: 23796953 Free PMC article. Review.
Cited by
- An enriched environment improves long-term functional outcomes in mice after intracerebral hemorrhage by mechanisms that involve the Nrf2/BDNF/glutaminase pathway.
Jia P, Wang J, Ren X, He J, Wang S, Xing Y, Chen D, Zhang X, Zhou S, Liu X, Yu S, Li Z, Jiang C, Zang W, Chen X, Wang J. Jia P, et al. J Cereb Blood Flow Metab. 2023 May;43(5):694-711. doi: 10.1177/0271678X221135419. Epub 2023 Jan 12. J Cereb Blood Flow Metab. 2023. PMID: 36635875 Free PMC article. - Mechanisms of Oxidative Stress and Therapeutic Targets following Intracerebral Hemorrhage.
Yao Z, Bai Q, Wang G. Yao Z, et al. Oxid Med Cell Longev. 2021 Feb 21;2021:8815441. doi: 10.1155/2021/8815441. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33688394 Free PMC article. Review. - Lymphocyte-Related Immunomodulatory Therapy with Siponimod (BAF-312) Improves Outcomes in Mice with Acute Intracerebral Hemorrhage.
Zhang Z, Li Y, Shi J, Zhu L, Dai Y, Fu P, Liu S, Hong M, Zhang J, Wang J, Jiang C. Zhang Z, et al. Aging Dis. 2023 Jun 1;14(3):966-991. doi: 10.14336/AD.2022.1102. Aging Dis. 2023. PMID: 37191423 Free PMC article. - Molecular, Pathological, Clinical, and Therapeutic Aspects of Perihematomal Edema in Different Stages of Intracerebral Hemorrhage.
Jiang C, Guo H, Zhang Z, Wang Y, Liu S, Lai J, Wang TJ, Li S, Zhang J, Zhu L, Fu P, Zhang J, Wang J. Jiang C, et al. Oxid Med Cell Longev. 2022 Sep 17;2022:3948921. doi: 10.1155/2022/3948921. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36164392 Free PMC article. Review. - Inhibition of neuronal ferroptosis protects hemorrhagic brain.
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J. Li Q, et al. JCI Insight. 2017 Apr 6;2(7):e90777. doi: 10.1172/jci.insight.90777. JCI Insight. 2017. PMID: 28405617 Free PMC article.
References
- Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 2007; 27: 894–908. - PubMed
- Gong C, Ennis SR, Hoff JT, et al. Inducible cyclooxygenase-2 expression after experimental intracerebral hemorrhage. Brain Res 2001; 901: 38–46. - PubMed
- Chu K, Jeong SW, Jung KH, et al. Celecoxib induces functional recovery after intracerebral hemorrhage with reduction of brain edema and perihematomal cell death. J Cereb Blood Flow Metab 2004; 24: 926–933. - PubMed
- Lee SH, Park HK, Ryu WS, et al. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicenter randomized controlled trial. Eur J Neurol 2013; 20: 1161–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous