YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing - PubMed (original) (raw)
YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing
Arupratan Das et al. J Biol Chem. 2016.
Abstract
Cell-cell contact inhibition and the mechanical environment of cells have both been shown to regulate YAP nuclear localization to modulate cell proliferation. Changes in cellular contractility by genetic, pharmacological, and matrix stiffness perturbations regulate YAP nuclear localization. However, because contractility and F-actin organization are interconnected cytoskeletal properties, it remains unclear which of these distinctly regulates YAP localization. Here we show that in the absence of cell-cell contact, actomyosin contractility suppresses YAP phosphorylation at Ser(112), however, neither loss of contractility nor increase in YAP phosphorylation is sufficient for its nuclear exclusion. We find that actin cytoskeletal integrity is essential for YAP nuclear localization, and can override phosphoregulation or contractility-mediated regulation of YAP nuclear localization. This actin-mediated regulation is conserved during mechanotransduction, as substrate compliance increased YAP phosphorylation and reduced cytoskeletal integrity leading to nuclear exclusion of both YAP and Ser(P)(112)-YAP. These data provide evidence for two actin-mediated pathways for YAP regulation; one in which actomyosin contractility regulates YAP phosphorylation, and a second that involves cytoskeletal integrity-mediated regulation of YAP nuclear localization independent of contractility. We suggest that in non-contact inhibited cells, this latter mechanism may be important in low stiffness regimes, such as may be encountered in physiological environments.
Keywords: F-actin; Hippo pathway; YAP; cell adhesion; contact inhibition; contractility; cytoskeleton; mechanotransduction; myosin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
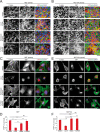
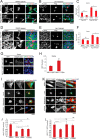
FIGURE 1.
In the absence of cell-cell contact, nuclear exclusion of YAP requires F-actin cytoskeletal integrity, but not ROCK-mediated myosin II contractility. Confocal immunofluorescence images of MEF (A) or human mammary epithelial cells (B, MCF10A) densely plated on fibronectin-coated coverslips and treated with the indicated agents for 2 h. F-actin was stained with phalloidin-488 (green), YAP with immunolocalization of α-YAP (red), and DNA with DAPI (blue). Maximum projections of confocal Z-stacks of sparsely plated MEFs (C) or MCF10A (E), on fibronectin-coated coverslips and treated with the indicated agents for 2 h. Immunofluorescence staining as in A and B. D and F, quantification of the nuclear/cytoplasmic ratio of YAP in the middle confocal slice of the nucleus. Scale bars 30 μm. Error bars are S.E. Statistical comparison done with Student's t test between pair of samples connected with lines on the column plots: **, p value < 0.005; _ns_, _p_ value > 0.05. One-way ANOVA tests were performed on group of data containing three or more than three data sets, p values from ANOVA tests are listed in Table 1.
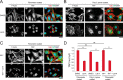
FIGURE 2.
In sparse cells, F-actin cytoskeletal integrity is required for YAP nuclear localization but not integrin signaling. Maximum projections of confocal Z-stacks of sparsely plated MEFs on fibronectin (A and C) and poly-
l
-lysine (B)-coated coverslips and treated with Lat-A (2 μ
m
), manganese(II) chloride (Mn2+, 1 m
m
), and Mn2+ along with Lat-A (2 μ
m
) as indicated. F-actin was stained with phalloidin-561 (red), YAP with immunolocalization of α-YAP (green), and DNA with DAPI (blue). D, quantification of the nuclear/cytoplasmic ratio of YAP as done as described in the legend to Fig. 1. Scale bars 30 μm. Error bars are S.E. Statistical comparison done with Student's t test between pair of samples connected with lines on the column plot: **, p value < 0.005; _ns_, _p_ value > 0.05. One-way ANOVA test was performed on D, p value from ANOVA test is listed in Table 1.
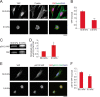
FIGURE 3.
In sparse cells, nuclear localization of YAP is promoted by F-actin independent of myosin II contractility and is not fully dependent on Ser112 phosphorylation. A, left, representative Western blots of whole cell lysates from MEFs grown on fibronectin-coated culture dishes and treated with DMSO, Lat-A (2 μ
m
), and blebbistatin (50 μ
m
) for 2 h and probed with antibodies to YAP or YAP phosphorylated on Ser112 (pS112-YAP). Right, quantification represents the ratio of integrated band intensity between Ser(P)112-YAP and YAP from background subtracted images and normalized with respect to DMSO control ratio. B, maximum intensity projections of confocal Z-stack immunofluorescence images of sparsely plated MEFs treated with the indicated agents for 2 h. YAP was immunostained with α-YAP (red), α-Ser(P)112-YAP (green), and DNA with DAPI (blue). Note that Ser(P)112-YAP was better detected with methanol fixation (used here), however, YAP was better detected with paraformaldehyde fixation (used in Fig. 1). C, quantification of the nuclear/cytoplasmic ratio of pS112-YAP in the middle confocal slice of the nucleus. D, representative Western blots of the nucleus (Nuc) and cytoplasmic (Cyt) fractions from MEFs grown on fibronectin-coated culture dishes with the indicated treatments for 2 h for the indicated proteins and probed with antibodies to YAP, Ser(P)112-YAP, and Histone H3. Scale bars 30 μm. Error bars are S.E. Statistical comparison done with Student's t test between pair of samples connected with lines on the column plots: *, p value < 0.05; **, _p_ value < 0.005; _ns_, _p_ value > 0.05. One-way ANOVA tests were performed on group of data containing three or more data sets, p values from ANOVA tests are listed in Table 1.
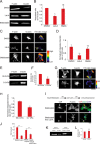
FIGURE 4.
Mechanosensation of soft ECM promotes nuclear exclusion of YAP and phospho-Ser112-YAP. A–F, MEFs were grown on fibronectin-coupled polyacrylamide hydrogels of 50.0 (stiff) and 0.5 kPa (soft) stiffness. A, maximum intensity projections of confocal Z-stack immunofluorescence images of YAP (red), phalloidin staining of F-actin (green), and DAPI staining of DNA (blue). B, quantification of the nuclear/cytoplasmic ratio of YAP in the middle confocal slice of the nucleus. C, representative Western blots from whole cell lysates of MEFs grown on fibronectin-coated soft and stiff substrates and probed with antibodies to YAP and YAP phosphorylated on Ser112 (pS112-YAP). D, quantification of the ratio of integrated band intensity between Ser(P)112-YAP and YAP from background-subtracted Western blot images and normalized with respect to 50.0 kPa. E, maximum intensity projections of confocal Z-stack immunofluorescence images of sparsely plated MEFs grown on fibronectin-coated stiff and soft substrates and fixed with methanol and immunostained to detect YAP (red), Ser(P)112-YAP (green), and stained with DAPI for DNA (blue). F, quantification of the nuclear/cytoplasmic ratio of Ser(P)112-YAP in the middle confocal slice of the nucleus. Scale bars 30 μm. Error bars are S.E., Statistical comparison done with Student's t test: *, p value < 0.05; **, p value < 0.005.
FIGURE 5.
F-actin promotes YAP nuclear localization in the absence of actomyosin contractility. A and E are representative Western blots of the Triton-insoluble (F-actin) and Triton-soluble (G-actin) fractions extracted from MEFs grown on fibronectin-coated culture dish treated with DMSO, Lat-A (2 μ
m
), and blebbistatin (50 μm) for 2 h (A) or grown on fibronectin-coupled polyacrylamide hydrogels of 50.0 (stiff) and 0.5 kPa (soft) stiffness (E) and probed with antibodies to actin. B and F, quantification represents the ratio of integrated band intensity between the F-actin and G-actin from background-subtracted Western blot images. The proportions of F- and G-actin were determined relative to the total actin content (total actin content = _F_s + _G_s signal = 100%; F = _F_s/_F_s + _G_s; G = _G_s/_F_s + _G_s), and these values were used to determine the F:G ratio (F/G = (_F_s/_F_s + _G_s)/(_G_s/_F_s + _G_s)). The F:G ratio for the DMSO condition was then used as the normalization control to compare the effects of perturbations (B) or to 50-kPa control ratio (F). C and G, sum projections of confocal Z-stacks of MEFs grown on fibronectin-coated culture dishes and treated with DMSO, Lat-A (2 μ
m
), and blebbistatin (50 μm) for 2 h (C) or grown on fibronectin-coupled polyacrylamide hydrogels of 55.0 kPa (stiff) and 0.7 kPa (soft) stiffness (G) and stained for F-actin (phalloidin-488, green) and G-actin (DNase I-594, red). The F:G-actin ratio images were generated using ImageJ as explained in (48). D and H, quantification represents the ratio of integrated fluorescence intensity measured from the background subtracted sum-projection of F- and G-actin images, normalized with respect to the average ratio of DMSO control (D) or 55 kPa control (H). S.E. and p values were measured from the non-normalized data set. I, above, experimental scheme for examining YAP localization in MEFs first treated with 100 μ
m
blebbistatin for 2 h followed by Lat-A (2 μ
m
) treatment for another 2 h (blebbistatin then Lat-A), and compared with 100 μ
m
blebbistatin treatment for 2 h alone. Maximum intensity projections of confocal Z-stack immunofluorescence images of sparsely plated MEFs grown on fibronectin-coated coverslips and were treated as explained above. F-actin was stained with phalloidin-488 (green), YAP with immunolocalization of α-YAP (red), and DNA with DAPI (blue). J, quantification of the nuclear/cytoplasmic ratio of YAP in the middle confocal slice of the nucleus. DMSO and Lat-A data are from Fig. 1_D. K,_ representative Western blot of the Triton-insoluble (F-actin) and Triton-soluble (G-actin) fractions extracted from MEFs grown sparsely and densely on fibronectin coated culture dish. L, quantification represents the ratio of integrated band intensity between the F-actin and G-actin from background-subtracted Western blot images and normalized with respect to sparse ratio. Scale bars 30 μm. Error bars are S.E., Statistical comparison was done with Student's t test between pair of samples connected with lines on the column plots: *, p value < 0.05; **, _p_ value < 0.005; _ns_, _p_ value > 0.05. One-way ANOVA tests were performed on a group of data containing three or more data sets, p values from ANOVA tests are listed in Table 1.
FIGURE 6.
Loss of cytoskeletal integrity significantly excludes constitutively active YAP from the nucleus. Maximum intensity projections of confocal Z-stacks of MEFs transfected with plasmids expressing FLAG-YAP and FLAG-YAP-8SA, cultured sparsely (A and B) or middle confocal slice of densely plated MEFs (D and E) on fibronectin-coated coverslips and treated with DMSO and Lat-A (2 μ
m
) as indicated. C and F, quantification of the nuclear/cytoplasmic ratio of FLAG in the middle confocal slice of the nucleus. F-actin stained with phalloidin-488 (green), FLAG-YAP with α-FLAG (red), and DNA with DAPI (blue). G–L, confocal images of MEFs transfected with plasmids expressing YAP1-GFP, or non-phosphorylatable _YAP1S112A_-GFP (green) of mouse origin and mApple-actin (red) that were densely plated on fibronectin coated coverslips for 18 h and fixed with paraformaldehyde (G). MEFs were transfected with plasmids expressing YAP1-GFP (I, green), non-phosphorylatable _YAP1S112A_-GFP (K, green) of mouse origin and mApple-actin (red) and grown on fibronectin-coated coverslips sparsely for 18 h followed by DMSO, Lat-A (2 μ
m
), blebbistatin (50 μ
m
), and Y-27632 (50 μ
m
) treatment for 2 h and formaldehyde fixation. YAP1 was detected by the GFP signal (green), actin by red fluorescent protein mApple (red), and DNA stained with DAPI (blue). H, J, and L, quantification of the nuclear/cytoplasmic ratio of YAP1-GFP in the middle confocal slice of the nucleus. Scale bars 30 μm. Error bars are S.E., Statistical comparison done with Student's t test between pairs of samples connected with lines on the column plots: *, p value < 0.05; **, _p_ value < 0.005; _ns_, _p_ value > 0.05. One-way ANOVA tests were performed on group of data containing three or more data sets, p values from ANOVA tests are listed in Table 1.
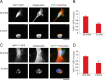
FIGURE 7.
ECM compliance-mediated regulation of YAP nuclear localization is predominantly regulated by F-actin-dependent, phosphorylation-independent pathway. MEFs were grown on fibronectin-coupled polyacrylamide hydrogels of 50.0 (stiff) and 0.5 kPa (soft) stiffness. YAP1wt-GFP (A, green) or non-phosphorylatable _YAP1S112A_-GFP (C, green) was detected by the GFP signal, actin by red fluorescent protein mApple (red), and DNA stained with DAPI (blue). * in C, marks the nuclei of an adjacent untransfected cell. B and D, quantification of the nuclear/cytoplasmic ratio of YAP1-GFP constructs in the middle confocal slice of the nucleus. Scale bars 30 μm. Error bars are S.E., Statistical comparison was done with Student's t test: **, p value < 0.005.
Similar articles
- F-actin dynamics regulates mammalian organ growth and cell fate maintenance.
Pocaterra A, Santinon G, Romani P, Brian I, Dimitracopoulos A, Ghisleni A, Carnicer-Lombarte A, Forcato M, Braghetta P, Montagner M, Galuppini F, Aragona M, Pennelli G, Bicciato S, Gauthier N, Franze K, Dupont S. Pocaterra A, et al. J Hepatol. 2019 Jul;71(1):130-142. doi: 10.1016/j.jhep.2019.02.022. Epub 2019 Mar 14. J Hepatol. 2019. PMID: 30878582 - Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts.
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Calvo F, et al. Nat Cell Biol. 2013 Jun;15(6):637-46. doi: 10.1038/ncb2756. Epub 2013 May 26. Nat Cell Biol. 2013. PMID: 23708000 Free PMC article. - Nuclear compression regulates YAP spatiotemporal fluctuations in living cells.
Koushki N, Ghagre A, Srivastava LK, Molter C, Ehrlicher AJ. Koushki N, et al. Proc Natl Acad Sci U S A. 2023 Jul 11;120(28):e2301285120. doi: 10.1073/pnas.2301285120. Epub 2023 Jul 3. Proc Natl Acad Sci U S A. 2023. PMID: 37399392 Free PMC article. - Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction.
Dupont S. Dupont S. Exp Cell Res. 2016 Apr 10;343(1):42-53. doi: 10.1016/j.yexcr.2015.10.034. Epub 2015 Oct 30. Exp Cell Res. 2016. PMID: 26524510 Review. - Control of cellular responses to mechanical cues through YAP/TAZ regulation.
Dasgupta I, McCollum D. Dasgupta I, et al. J Biol Chem. 2019 Nov 15;294(46):17693-17706. doi: 10.1074/jbc.REV119.007963. Epub 2019 Oct 8. J Biol Chem. 2019. PMID: 31594864 Free PMC article. Review.
Cited by
- A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes.
Scott KE, Fraley SI, Rangamani P. Scott KE, et al. Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2021571118. doi: 10.1073/pnas.2021571118. Epub 2021 May 14. Proc Natl Acad Sci U S A. 2021. PMID: 33990464 Free PMC article. - YAP mechanotransduction under cyclic mechanical stretch loading for mesenchymal stem cell osteogenesis is regulated by ROCK.
Kim E, Riehl BD, Bouzid T, Yang R, Duan B, Donahue HJ, Lim JY. Kim E, et al. Front Bioeng Biotechnol. 2024 Jan 11;11:1306002. doi: 10.3389/fbioe.2023.1306002. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38274006 Free PMC article. - Insights gained from computational modeling of YAP/TAZ signaling for cellular mechanotransduction.
Jafarinia H, Khalilimeybodi A, Barrasa-Fano J, Fraley SI, Rangamani P, Carlier A. Jafarinia H, et al. NPJ Syst Biol Appl. 2024 Aug 15;10(1):90. doi: 10.1038/s41540-024-00414-9. NPJ Syst Biol Appl. 2024. PMID: 39147782 Free PMC article. Review. - Mechanical Stretch Induced Skin Regeneration: Molecular and Cellular Mechanism in Skin Soft Tissue Expansion.
Guo Y, Song Y, Xiong S, Wang T, Liu W, Yu Z, Ma X. Guo Y, et al. Int J Mol Sci. 2022 Aug 25;23(17):9622. doi: 10.3390/ijms23179622. Int J Mol Sci. 2022. PMID: 36077018 Free PMC article. Review. - Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells.
Darnell M, O'Neil A, Mao A, Gu L, Rubin LL, Mooney DJ. Darnell M, et al. Proc Natl Acad Sci U S A. 2018 Sep 4;115(36):E8368-E8377. doi: 10.1073/pnas.1802568115. Epub 2018 Aug 17. Proc Natl Acad Sci U S A. 2018. PMID: 30120125 Free PMC article.
References
- Wu S., Huang J., Dong J., and Pan D. (2003) Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445–456 - PubMed
- Polesello C., and Tapon N. (2007) Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864–1870 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases