Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior - PubMed (original) (raw)
Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior
Yuyan Cheng et al. Brain Behav Immun. 2016 Mar.
Abstract
Most psychiatric and neurological diseases are exacerbated by stress. Because this may partially result from stress-induced inflammation, we examined factors involved in this stress response. After a paradigm of inescapable foot shock stress that causes learned helplessness depression-like behavior, eighteen cytokines and chemokines increased in mouse hippocampus, peaking 6-12h after stress. A 24h prior pre-conditioning stress accelerated the rate of stress-induced hippocampal cytokine and chemokine increases, with most reaching peak levels after 1-3h, often without altering the maximal levels. Toll-like receptor 4 (TLR4) was involved in this response because most stress-induced hippocampal cytokines and chemokines were attenuated in TLR4 knockout mice. Stress activated glycogen synthase kinase-3 (GSK3) in wild-type mouse hippocampus, but not in TLR4 knockout mice. Administration of the antidepressant fluoxetine or the GSK3 inhibitor TDZD-8 reduced the stress-induced increases of most hippocampal cytokines and chemokines. Stress increased hippocampal levels of the danger-associated molecular pattern (DAMP) protein high mobility group box 1 (HMGB1), activated the inflammatory transcription factor NF-κB, and the NLRP3 inflammasome. Knockdown of HMGB1 blocked the acceleration of cytokine and chemokine increases in the hippocampus caused by two successive stresses. Fluoxetine treatment blocked stress-induced up-regulation of HMGB1 and subsequent NF-κB activation, whereas TDZD-8 administration attenuated NF-κB activation downstream of HMGB1. To test if stress-induced cytokines and chemokines contribute to depression-like behavior, the learned helplessness model was assessed. Antagonism of TNFα modestly reduced susceptibility to learned helplessness induction, whereas TLR4 knockout mice were resistant to learned helplessness. Thus, stress-induces a broad inflammatory response in mouse hippocampus that involves TLR4, GSK3, and downstream inflammatory signaling, and these stress responses contribute to susceptibility to depression-like behavior in mice.
Keywords: Depression; Fluoxetine; Glycogen synthase kinase-3; Neuroinflammation; Stress; Toll-like receptor 4.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
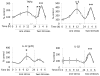
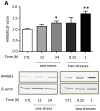
Figure 1. Cytokines and chemokines in the brain after one and two stresses
Mice were subjected to one or two daily sessions of inescapable foot shocks. Hippocampi were collected 1, 6, 12, and 24 hr after a single session of inescapable foot shocks, or 0.25, 1, 3, and 6 hr after two daily sessions of inescapable foot shocks, followed by measurements of TNFα (n = 6–10), IL-6 (n = 4–7), IL-12(p70) (n= 4–7), and IL-1β (n = 3–4) in the hippocampus by ELISA. Values are means±SEM; Vertical dashed line separates the mice subjected to one stress (left) and those subjected to two stresses (right); *p < 0.05 compared to non-stressed control (0 hr) group; **p < 0.05 compared to 24 hr after the first stress (one-way ANOVA).
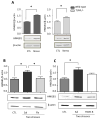
Figure 2. Role of TLR4 in the response to stress
(A) TLR4 mRNA levels 6, 12, and 24 hr following administration of a single session of inescapable foot shocks. 18s mRNA was used as an internal control. Bars represent means±SEM; n = 3–5 in each group; *p < 0.05 compared to non-stressed control (CTL) group (one-way ANOVA). (B) Wild-type and TLR4−/− mice were subjected to one or two daily sessions of inescapable foot shocks followed by measurements of cytokines and chemokines in the hippocampus by multiplex analysis. Hippocampi were collected 6, 12, and 24 hr after a single session of inescapable foot shocks, or 1 and 3 hr after two sessions of inescapable foot shocks. Hippocampi from 3–4 mice in each group were pooled for multiplex analysis.
Figure 2. Role of TLR4 in the response to stress
(A) TLR4 mRNA levels 6, 12, and 24 hr following administration of a single session of inescapable foot shocks. 18s mRNA was used as an internal control. Bars represent means±SEM; n = 3–5 in each group; *p < 0.05 compared to non-stressed control (CTL) group (one-way ANOVA). (B) Wild-type and TLR4−/− mice were subjected to one or two daily sessions of inescapable foot shocks followed by measurements of cytokines and chemokines in the hippocampus by multiplex analysis. Hippocampi were collected 6, 12, and 24 hr after a single session of inescapable foot shocks, or 1 and 3 hr after two sessions of inescapable foot shocks. Hippocampi from 3–4 mice in each group were pooled for multiplex analysis.
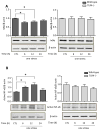
Figure 3. Stress activates GSK3 in mouse hippocampus
Serine-phosphorylated (A, C) GSK3α and (B, D) GSK3β, and total GSK3α/β (GSK3) were measured in mouse hippocampus at the indicated times after one or two daily inescapable foot shock stresses in wild-type (A: n = 4–7; B: n = 7–9) and TLR4−/− mice (C and D: n = 3–4). Quantitation of GSK3β includes both the main isoform and the slightly slower migrating alternatively spliced GSK3β2 variant. Bars represent means±SEM; *p < 0.05 compared to non-stressed control (CTL) group; **p < 0.05 compared to 24 hr after the first stress (one-way ANOVA).
Figure 3. Stress activates GSK3 in mouse hippocampus
Serine-phosphorylated (A, C) GSK3α and (B, D) GSK3β, and total GSK3α/β (GSK3) were measured in mouse hippocampus at the indicated times after one or two daily inescapable foot shock stresses in wild-type (A: n = 4–7; B: n = 7–9) and TLR4−/− mice (C and D: n = 3–4). Quantitation of GSK3β includes both the main isoform and the slightly slower migrating alternatively spliced GSK3β2 variant. Bars represent means±SEM; *p < 0.05 compared to non-stressed control (CTL) group; **p < 0.05 compared to 24 hr after the first stress (one-way ANOVA).
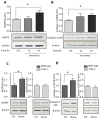
Figure 4. Pretreatment with fluoxetine or TDZD-8 attenuates stress-induced cytokines and chemokines in mouse hippocampus
Following two weeks of daily treatment with fluoxetine (Flx), TDZD-8, or saline (Sal), mice were subjected to two daily sessions of inescapable foot shocks and after 3 hr cytokines and chemokines in the hippocampus were measured by (A) ELISA or (B) multiplex. ELISA values are means±SEM; n = 5–6 mice in each group; *p < 0.05 compared to non-stressed control (CTL) mice or to mice treated with saline (one-way ANOVA). Hippocampi from 3–4 mice in each group were pooled for multiplex analysis. (C) A diagram summarizing hippocampal cytokine and chemokine changes affected by two daily stresses stress. Cytokines and chemokines within the outer circle were increased by stress, and the labeled circles show the cytokines and chemokines that were decreased by TLR4 deficiency, fluoxetine treatment, and GSK3 inhibition.
Figure 4. Pretreatment with fluoxetine or TDZD-8 attenuates stress-induced cytokines and chemokines in mouse hippocampus
Following two weeks of daily treatment with fluoxetine (Flx), TDZD-8, or saline (Sal), mice were subjected to two daily sessions of inescapable foot shocks and after 3 hr cytokines and chemokines in the hippocampus were measured by (A) ELISA or (B) multiplex. ELISA values are means±SEM; n = 5–6 mice in each group; *p < 0.05 compared to non-stressed control (CTL) mice or to mice treated with saline (one-way ANOVA). Hippocampi from 3–4 mice in each group were pooled for multiplex analysis. (C) A diagram summarizing hippocampal cytokine and chemokine changes affected by two daily stresses stress. Cytokines and chemokines within the outer circle were increased by stress, and the labeled circles show the cytokines and chemokines that were decreased by TLR4 deficiency, fluoxetine treatment, and GSK3 inhibition.
Figure 4. Pretreatment with fluoxetine or TDZD-8 attenuates stress-induced cytokines and chemokines in mouse hippocampus
Following two weeks of daily treatment with fluoxetine (Flx), TDZD-8, or saline (Sal), mice were subjected to two daily sessions of inescapable foot shocks and after 3 hr cytokines and chemokines in the hippocampus were measured by (A) ELISA or (B) multiplex. ELISA values are means±SEM; n = 5–6 mice in each group; *p < 0.05 compared to non-stressed control (CTL) mice or to mice treated with saline (one-way ANOVA). Hippocampi from 3–4 mice in each group were pooled for multiplex analysis. (C) A diagram summarizing hippocampal cytokine and chemokine changes affected by two daily stresses stress. Cytokines and chemokines within the outer circle were increased by stress, and the labeled circles show the cytokines and chemokines that were decreased by TLR4 deficiency, fluoxetine treatment, and GSK3 inhibition.
Figure 5. Stress increases hippocampal HMGB1 that accelerates increases in stress-induced cytokines and chemokines
(A) Hippocampal HMGB1 levels in wild-type mice after administration of a single or two sessions of inescapable foot shocks at the indicated times. n = 5–8 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed; **p<0.05 compared to 24 hr after the first stress (one-way ANOVA). (B, C) HMGB1 siRNA or scrambled siRNA was administered intranasally to mice 1 hr after the first of two daily sessions of inescapable foot shocks, and hippocampi were collected 3 and 12 hr after the second session. (B) Hippocampal HMGB1 levels were measured 3 hr after two stresses by immunoblotting hippocampal extracts using β-actin as a loading control. Bars represent means±SEM; n = 4–6 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed or mice treated with scrambled siRNA (one-way ANOVA). (C) Measurements of hippocampal TNFα, IL-6, IL-12(p70), and IL-1β by ELISA at 3 and 12 hr after two stresses. Values are means±SEM; n = 4–6 mice per group; *p < 0.05 compared to non-stressed control (0 hr) mice (one-way ANOVA).
Figure 5. Stress increases hippocampal HMGB1 that accelerates increases in stress-induced cytokines and chemokines
(A) Hippocampal HMGB1 levels in wild-type mice after administration of a single or two sessions of inescapable foot shocks at the indicated times. n = 5–8 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed; **p<0.05 compared to 24 hr after the first stress (one-way ANOVA). (B, C) HMGB1 siRNA or scrambled siRNA was administered intranasally to mice 1 hr after the first of two daily sessions of inescapable foot shocks, and hippocampi were collected 3 and 12 hr after the second session. (B) Hippocampal HMGB1 levels were measured 3 hr after two stresses by immunoblotting hippocampal extracts using β-actin as a loading control. Bars represent means±SEM; n = 4–6 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed or mice treated with scrambled siRNA (one-way ANOVA). (C) Measurements of hippocampal TNFα, IL-6, IL-12(p70), and IL-1β by ELISA at 3 and 12 hr after two stresses. Values are means±SEM; n = 4–6 mice per group; *p < 0.05 compared to non-stressed control (0 hr) mice (one-way ANOVA).
Figure 5. Stress increases hippocampal HMGB1 that accelerates increases in stress-induced cytokines and chemokines
(A) Hippocampal HMGB1 levels in wild-type mice after administration of a single or two sessions of inescapable foot shocks at the indicated times. n = 5–8 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed; **p<0.05 compared to 24 hr after the first stress (one-way ANOVA). (B, C) HMGB1 siRNA or scrambled siRNA was administered intranasally to mice 1 hr after the first of two daily sessions of inescapable foot shocks, and hippocampi were collected 3 and 12 hr after the second session. (B) Hippocampal HMGB1 levels were measured 3 hr after two stresses by immunoblotting hippocampal extracts using β-actin as a loading control. Bars represent means±SEM; n = 4–6 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed or mice treated with scrambled siRNA (one-way ANOVA). (C) Measurements of hippocampal TNFα, IL-6, IL-12(p70), and IL-1β by ELISA at 3 and 12 hr after two stresses. Values are means±SEM; n = 4–6 mice per group; *p < 0.05 compared to non-stressed control (0 hr) mice (one-way ANOVA).
Figure 6. Stress-induced increases in hippocampal HMGB1 levels is blocked by fluoxetine treatment
(A) Hippocampal HMGB1 levels 1 hr after two sessions of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice in each group; *p < 0.05 compared with control (CTL) mice that were not stressed (Student’s t test). Following two weeks of daily treatment with saline (Sal), (B) fluoxetine (Flx), or (C) TDZD-8, mice were subjected to two daily sessions of inescapable foot shocks and HMGB1 levels were measured by immunoblotting hippocampal extracts using β-actin as a loading control. n = 5–6 mice in each group; *p < 0.05 (one-way ANOVA).
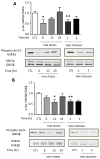
Figure 7. Effects of TLR4 deficiency and pretreatment with fluoxetine or TDZD-8 on stress-induced hippocampal IκBα and p65-NF-κB levels
Hippocampal (A) IκBα and (B) p65-NF-κB levels 6, 12 and 24 hr after administration of a single session of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice in each group; *p < 0.05 compared with control (CTL) mice that were not stressed (one-way ANOVA). Mice were pretreated for two weeks with fluoxetine (Flx), TDZD-8, or saline (Sal) and subjected to two daily sessions of inescapable foot shocks followed by measuring hippocampal (C) IκBα and (D) active p65-NF-κB levels. n = 5–6 in each group; *p < 0.05 compared with saline-treated mice (one-way ANOVA).
Figure 7. Effects of TLR4 deficiency and pretreatment with fluoxetine or TDZD-8 on stress-induced hippocampal IκBα and p65-NF-κB levels
Hippocampal (A) IκBα and (B) p65-NF-κB levels 6, 12 and 24 hr after administration of a single session of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice in each group; *p < 0.05 compared with control (CTL) mice that were not stressed (one-way ANOVA). Mice were pretreated for two weeks with fluoxetine (Flx), TDZD-8, or saline (Sal) and subjected to two daily sessions of inescapable foot shocks followed by measuring hippocampal (C) IκBα and (D) active p65-NF-κB levels. n = 5–6 in each group; *p < 0.05 compared with saline-treated mice (one-way ANOVA).
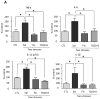
Figure 8. Effects of stress on NLRP3 inflammasome activation in the hippocampus
Hippocampal (A) NLRP3 and (B) active p20 caspase-1 levels were measured 12 and 24 hr after administration of a single session of inescapable foot shocks in wild-type mice. n = 6–8 mice in each group; *p < 0.05 compared with mice that were not stressed (one-way ANOVA). Hippocampal (A) NLRP3 and (B) active p20 caspase-1 levels were measured 1 hr after two daily sessions of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice p group; *p < 0.05 compared with mice that were not stressed (Student’s t test).
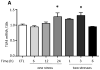
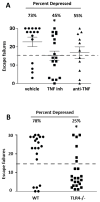
Figure 9. TLR4−/− mice are resistant to learned helplessness
(A) Learned helplessness was evaluated in wild-type mice following treatment with a small molecule TNFα inhibitor, Vax-ATC1, the anti-TNFα etanercept, or vehicle every other day for a week. n = 11–18 in each group. *p < 0.05 compared with mice treated with vehicle (Mann Whitney test). (B) Wild-type (WT) and TLR4−/− mice were subjected to the learned helplessness paradigm. Each point represents the number of failures to escape for a single mouse. Bars represent means ± SEM. n = 20–23 mice in each group. *p < 0.05 compared with wild-type mice (Student’s t test).
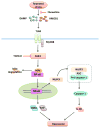
Figure 10. Hypothetical scheme of the signaling activated by stress
Stress activates TLR4, possibly through DAMP molecules, leading to the degradation of IκBα and the activation of NF-κB, which induces increased levels of several cytokines and chemokines including TNFα and IL-6. A pre-conditioning stress activates TLR4 signaling and increases expression of NLRP3, possibly through NF-κB activation (Bauernfeind et al., 2009; Guo et al., 2015), followed by assembly of the NLRP3 inflammasome and activation of caspase-1 leading to increased IL-1β production. A prior stress also increases the DAMP HMGB1, which may activate TLR4 to accelerate the rate of cytokine and chemokine increases after the second stress. Fluoxetine reduces stress-induced neuroinflammation and dampens stress-induced increases in HMGB1 and activation of NF-κB, and inhibition of GSK3 by TDZD-8 reduces stress-induced cytokines and chemokines and inhibits activation of NF-κB. MyD88 = Myeloid differentiation primary response gene 88; ASC = Apoptosis-associated speck-like protein containing a CARD.
Similar articles
- TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice.
Cheng Y, Desse S, Martinez A, Worthen RJ, Jope RS, Beurel E. Cheng Y, et al. Brain Behav Immun. 2018 Mar;69:556-567. doi: 10.1016/j.bbi.2018.02.003. Epub 2018 Feb 13. Brain Behav Immun. 2018. PMID: 29452218 Free PMC article. - HMGB1 was negatively regulated by HSF1 and mediated the TLR4/MyD88/NF-κB signal pathway in asthma.
Shang L, Wang L, Shi X, Wang N, Zhao L, Wang J, Liu C. Shang L, et al. Life Sci. 2020 Jan 15;241:117120. doi: 10.1016/j.lfs.2019.117120. Epub 2019 Dec 9. Life Sci. 2020. PMID: 31825792 - HMGB1-mediated differential response on hippocampal neurotransmitter disorder and neuroinflammation in adolescent male and female mice following cold exposure.
Xu B, Zang SC, Li SZ, Guo JR, Wang JF, Wang D, Zhang LP, Yang HM, Lian S. Xu B, et al. Brain Behav Immun. 2019 Feb;76:223-235. doi: 10.1016/j.bbi.2018.11.313. Epub 2018 Nov 23. Brain Behav Immun. 2019. PMID: 30476565 - Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.
Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Zhang P, et al. Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review. - The Pathologic Role of Toll-Like Receptor 4 in Prostate Cancer.
Ou T, Lilly M, Jiang W. Ou T, et al. Front Immunol. 2018 Jun 6;9:1188. doi: 10.3389/fimmu.2018.01188. eCollection 2018. Front Immunol. 2018. PMID: 29928275 Free PMC article. Review.
Cited by
- Fructus Arctii Mitigates Depressive Disorder via the Let-7e-Modulated Toll-Like Receptor (TLR) Signaling Pathway.
Zhang W, Zhou Q. Zhang W, et al. Brain Behav. 2024 Nov;14(11):e70132. doi: 10.1002/brb3.70132. Brain Behav. 2024. PMID: 39538967 Free PMC article. - Molecular signatures of astrocytes and microglia maladaptive responses to acute stress are rescued by a single administration of ketamine in a rodent model of PTSD.
Valenza M, Facchinetti R, Torazza C, Ciarla C, Bronzuoli MR, Balbi M, Bonanno G, Popoli M, Steardo L, Milanese M, Musazzi L, Bonifacino T, Scuderi C. Valenza M, et al. Transl Psychiatry. 2024 May 25;14(1):209. doi: 10.1038/s41398-024-02928-6. Transl Psychiatry. 2024. PMID: 38796504 Free PMC article. - The Emerging Role of Human Gut Bacteria Extracellular Vesicles in Mental Disorders and Developing New Pharmaceuticals.
Louka E, Koumandou VL. Louka E, et al. Curr Issues Mol Biol. 2024 May 15;46(5):4751-4767. doi: 10.3390/cimb46050286. Curr Issues Mol Biol. 2024. PMID: 38785554 Free PMC article. Review. - New Advances in the Pharmacology and Toxicology of Lithium: A Neurobiologically Oriented Overview.
Bortolozzi A, Fico G, Berk M, Solmi M, Fornaro M, Quevedo J, Zarate CA Jr, Kessing LV, Vieta E, Carvalho AF. Bortolozzi A, et al. Pharmacol Rev. 2024 May 2;76(3):323-357. doi: 10.1124/pharmrev.120.000007. Pharmacol Rev. 2024. PMID: 38697859 Free PMC article. Review. - Fluoxetine attenuates stress-induced depression-like behavior due to decrease in pro-inflammatory cytokines in male rats.
Nabirumbi R, Onohuean H, Drago KC, Alagbonsi AI, Adedeji AA. Nabirumbi R, et al. Sci Prog. 2024 Jan-Mar;107(1):368504241234786. doi: 10.1177/00368504241234786. Sci Prog. 2024. PMID: 38490226 Free PMC article.
References
- Abdel-Salam OM, Baiuomy AR, Arbid MS. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacological Research. 2004;49:119–131. - PubMed
- Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, Cordero MD. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014;36:111–117. - PubMed
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Anisman H, Merali Z, Hayley S. Sensitization associated with stressors and cytokine treatments. Brain Behav Immun. 2003;17:86–93. - PubMed
- Audet M, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun. 2011;25:1197–1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH104656/MH/NIMH NIH HHS/United States
- MH038752/MH/NIMH NIH HHS/United States
- R01 MH095380/MH/NIMH NIH HHS/United States
- MH090236/MH/NIMH NIH HHS/United States
- K99 MH090236/MH/NIMH NIH HHS/United States
- MH095380/MH/NIMH NIH HHS/United States
- R56 MH038752/MH/NIMH NIH HHS/United States
- R01 MH104656/MH/NIMH NIH HHS/United States
- R00 MH090236/MH/NIMH NIH HHS/United States
- R01 MH038752/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases