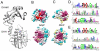Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm - PubMed (original) (raw)
Review
Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm
TuAnh Ngoc Huynh et al. Curr Opin Microbiol. 2016 Apr.
Abstract
Bacteria that synthesize c-di-AMP also encode several mechanisms for controlling c-di-AMP levels within the cytoplasm. One major class of phosphodiesterases comprises GdpP and DhhP homologs, which degrade c-di-AMP into the linear molecule 5'-pApA or AMP by the DHH-DHHA1 domain. The other major class comprises PgpH homologs, which degrade c-di-AMP by the HD domain. Both GdpP and PgpH harbor sensory domains, likely to regulate c-di-AMP hydrolysis activity in response to signal input. As another possible mechanism for controlling cytoplasmic c-di-AMP levels, bacteria also secrete c-di-AMP via multidrug resistance transporters, as demonstrated for Listeria monocytogenes. Mutants that accumulate high c-di-AMP levels, by deletion of phosphodiesterases or multidrug resistance transporters, exhibit aberrant physiology, growth defects, and attenuated virulence in infection.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Protein components of c-di-AMP depletion in the bacterial cytoplasm. PgpH and GdpP have sensory domains for integration of environmental and intracellular signals into enzymatic hydrolysis of c-di-AMP. DhhP (not shown) has a DHH-DHHA1 catalytic domain, but without accessory domains. Both PgpH and GdpP activities are inhibited by ppGpp. Heme-bound GdpP is activated by nitric oxide. Expression of MDRs is regulated by transcriptional factors of the MarR and TetR families. Small molecules such as bile acids influence DNA-binding activity of the TetR-like repressor BrtA and lead to transcriptional induction of MDRs. Enhanced MDR expression results in elevated secretion of bile acids and c-di-AMP.
Figure 2
Structural and sequence comparison of GdpP and DhhP catalytic domains. A – Crystal structure of the M. smegmatis DhhP (MSMEG_2630, PDB ID: 4LS9), also characterized as an NrnA oligoribonuclease. A metal ion is shown as a blue ball. A structural model of the GdpP DHH-DHHA1 domain, generated by the Phyre2 server, shows essentially the same fold, and is well aligned with NrnA. B and C – Comparison of conserved amino acids within the putative substrate recognition surface of the DHHA1 domain (top) and the metal binding site within the DHH domain (bottom). Similarly conserved residues are shown in magenta. GdpP also exhibits an additional conserved patch, shown in red, predicted for c-di-AMP binding by the Swissdock server. D - Sequence logos illustrating conserved residues of the GdpP DHH domain (top row) and DHHA1 domain (second and third rows), with conserved residues predicted for c-di-AMP binding labeled by a red bar. Residue 9 corresponds to Ile-341 in the B. subtilis GdpP sequence, residue 242 corresponds to Ile-574. E – Similar to D, but for DhhP homologs.
Figure 3
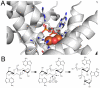
A- The c-di-AMP binding pocket in the HD domain active site of PgpH. His and Asp residues coordinate two metal ions (orange spheres). The phosphate of c-di-AMP coordinates these metal ions and hydrolysis occurs via a nucleophilic water molecule (red sphere) that is bridging between the metal ions. B- HD-domain catalytic mechanism for the phosphodiesterase reaction. Activated hydroxide group from the bridging water attacks the scissile phosphorous atom, with a 3’-hydroxyl as the leaving group, generating the linear product 5’-pApA. DHH-DHHA1 domains likely exhibit bi-nuclear active sites with a similar catalytic mechanism.
Similar articles
- An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence.
Huynh TN, Luo S, Pensinger D, Sauer JD, Tong L, Woodward JJ. Huynh TN, et al. Proc Natl Acad Sci U S A. 2015 Feb 17;112(7):E747-56. doi: 10.1073/pnas.1416485112. Epub 2015 Jan 12. Proc Natl Acad Sci U S A. 2015. PMID: 25583510 Free PMC article. - An Essential Poison: Synthesis and Degradation of Cyclic Di-AMP in Bacillus subtilis.
Gundlach J, Mehne FM, Herzberg C, Kampf J, Valerius O, Kaever V, Stülke J. Gundlach J, et al. J Bacteriol. 2015 Oct;197(20):3265-74. doi: 10.1128/JB.00564-15. Epub 2015 Aug 3. J Bacteriol. 2015. PMID: 26240071 Free PMC article. - Making and Breaking of an Essential Poison: the Cyclases and Phosphodiesterases That Produce and Degrade the Essential Second Messenger Cyclic di-AMP in Bacteria.
Commichau FM, Heidemann JL, Ficner R, Stülke J. Commichau FM, et al. J Bacteriol. 2018 Dec 7;201(1):e00462-18. doi: 10.1128/JB.00462-18. Print 2019 Jan 1. J Bacteriol. 2018. PMID: 30224435 Free PMC article. Review. - A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP.
Commichau FM, Dickmanns A, Gundlach J, Ficner R, Stülke J. Commichau FM, et al. Mol Microbiol. 2015 Jul;97(2):189-204. doi: 10.1111/mmi.13026. Epub 2015 May 9. Mol Microbiol. 2015. PMID: 25869574 Review.
Cited by
- The Many Roles of the Bacterial Second Messenger Cyclic di-AMP in Adapting to Stress Cues.
Zarrella TM, Bai G. Zarrella TM, et al. J Bacteriol. 2020 Dec 7;203(1):e00348-20. doi: 10.1128/JB.00348-20. Print 2020 Dec 7. J Bacteriol. 2020. PMID: 32839175 Free PMC article. Review. - Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate.
Athukoralage JS, Rouillon C, Graham S, Grüschow S, White MF. Athukoralage JS, et al. Nature. 2018 Oct;562(7726):277-280. doi: 10.1038/s41586-018-0557-5. Epub 2018 Sep 19. Nature. 2018. PMID: 30232454 Free PMC article. - Molecular Characterization of Equine Staphylococcus aureus Isolates Exhibiting Reduced Oxacillin Susceptibility.
Scholtzek AD, Hanke D, Walther B, Eichhorn I, Stöckle SD, Klein KS, Gehlen H, Lübke-Becker A, Schwarz S, Feßler AT. Scholtzek AD, et al. Toxins (Basel). 2019 Sep 13;11(9):535. doi: 10.3390/toxins11090535. Toxins (Basel). 2019. PMID: 31540335 Free PMC article. - NrnA Is a Linear Dinucleotide Phosphodiesterase with Limited Function in Cyclic Dinucleotide Metabolism in Listeria monocytogenes.
Gall AR, Hsueh BY, Siletti C, Waters CM, Huynh TN. Gall AR, et al. J Bacteriol. 2022 Jan 18;204(1):e0020621. doi: 10.1128/JB.00206-21. Epub 2021 Oct 18. J Bacteriol. 2022. PMID: 34662239 Free PMC article.
References
- Corrigan RM, Grundling A. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol. 2013;11:513–524. - PubMed
- Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J Biol Chem. 2010;285:473–482. ** Of outstanding interest: The first and most extensive biochemical analysis of GdpP phosphodiesterase
- Ye M, Zhang JJ, Fang X, Lawlis GB, Troxell B, Zhou Y, Gomelsky M, Lou Y, Yang XF. DhhP, a c-di-AMP phosphodiesterase of Borrelia burgdorferi, is essential for cell growth and virulence. Infect Immun. 2014 * Of special interest: A demonstration that toxic c-di-AMP levels prevent bacterial growth and confer pleotropic phenotypes
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI108698/AI/NIAID NIH HHS/United States
- AI108698/AI/NIAID NIH HHS/United States
- AI116669/AI/NIAID NIH HHS/United States
- T32 AI055396/AI/NIAID NIH HHS/United States
- R01 AI116669/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials