IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3 - PubMed (original) (raw)
IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3
Avanti Desai et al. J Allergy Clin Immunol. 2016 Jun.
Abstract
Background: IL-6, levels of which are reported to be increased in association with mastocytosis, asthma, and urticaria, is used in conjunction with stem cell factor to generate CD34(+) cell-derived primary human mast cell (HuMC) cultures. Despite these associations, the effects on and mechanisms by which prolonged exposure to IL-6 alters HuMC numbers and function are not well understood.
Objectives: We sought to study the effect of IL-6 on HuMC function, the mechanisms by which IL-6 exerts its effects, and the relationship of these findings to mastocytosis.
Methods: HuMCs were cultured in stem cell factor with or without IL-6. Responses to FcεRI aggregation and expression of proteases and receptors, including the soluble IL-6 receptor (sIL-6R), were then quantitated. Epigenetic changes in suppressor of cytokine signaling 3 (SOCS3) were determined by using methylation-specific PCR. Serum samples from healthy control subjects and patients with mastocytosis were assayed for IL-6, tryptase, and sIL-6R.
Results: IL-6 enhanced mast cell (MC) proliferation, maturation, and reactivity after FcεRI aggregation. IL-6 reduced expression of SOCS3, which correlated with methylation of the SOCS3 promoter and increased expression and activation of signal transducer and activator of transcription 3. IL-6 also suppressed constitutive production of sIL-6R, and serum levels of sIL-6R were similarly reduced in patients with mastocytosis.
Conclusion: IL-6 increases MC proliferation and formation of a more reactive phenotype enabled by suppressing proteolytic cleavage of sIL-6R from IL-6R and downregulation of the SOCS3 autoinhibitory pathway. We suggest IL-6 blockade might ameliorate MC-related symptoms and pathology in patients with MC-related diseases associated with increased IL-6 levels, including mastocytosis.
Keywords: IL-6; Mast cells; mastocytosis; signal transducer and activator of transcription 3; stem cell factor; suppressor of cytokine signaling 3.
Published by Elsevier Inc.
Conflict of interest statement
Authors state no conflict of interest.
Figures
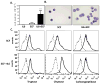
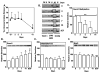
FIG 1. IL-6 enhances proliferation, alters cell morphology, and increases chymase content in HuMCs
HuMCs were cultured for 7 weeks in SCF, with or without IL6, or IL6 alone (100 ng/ml of each). (A) Mean cell count +/− SEM (cultures from five donors, *** P<0.001). (B) Cytocentrifuged preparations of cells stained with toluidine blue. (C) Tryptase, chymase and carboxypeptidase content by flow cytometry. Representative results from one of three donors are shown in B and C.
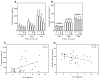
FIG 2. IL-6 enhances HuMC degranulation and cytokine production
(A and B) SA-induced release of β-hexosaminidase from HuMCs cultured in 100 ng/ml SCF and the indicated concentrations of IL-6 (A, n=7; B. n=1). (C–E) GMCSF and IL-8 production by HuMCs cultured in SCF (100 ng/ml) with or without IL-6 (100 ng/ml or as indicated) in response to SA and SCF (10 ng/ml each) (C, n=2; D, n=3; E, n=1). (F) Chemotactic response to 10 ng/ml SCF (n=3). Values are mean ± SEM (A, C, D, and F) or of replicate determinations of one HuMC donor (B and E). *P<0.05, **P<0.01, ***P<0.001.
FIG 3. Effect of IL-6 on release of sIL-6R and sgp130 from cultured HuMCs and in patients with mastocytosis
(A and B) Release of sIL-6R (n=3) and sgp130 (n=4) from SCF-conditioned HuMCs after transfer to medium containing SCF and IL-6; concentrations and times of exposure to IL-6 are indicated. Positive and negative correlations between serum IL-6 and MC tryptase (C) or sIL-6R and IL-6 (D) in patients with mastocytosis. Mean values ± SEM and significant differences are shown: *P<0.05, **P<0.01, ***P<0.001.
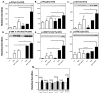
FIG 4. Increased FcεRI-mediated phosphorylation of signaling proteins in IL-6 conditioned HuMCs
HuMCs grown in SCF in the absence (open columns) or presence (solid columns) of IL-6 were stimulated or not with SA or SA with SCF (10 ng/ml of each) for 2 minutes. Representative immunoblots as well as the mean ± SEM values of densitometric data for the indicated proteins and phosphorylated proteins for HuMCs from three donors are shown. Significant differences, * P<0.05, ** P<0.01, *** P<0.001.
FIG 5. Effects of IL-6 on SOCS3, methylation of SOCS3 promoter, STAT3, and phosphorylated STAT3
SCF-conditioned HuMCs were exposed to IL-6 (100 ng/ml) for the hours indicated or maintained in SCF and IL-6 continuously. (A) Representative immunoblot and densitometric data for levels of SOCS3 under both conditions (n=3). (B) Representative gel image obtained for methylation of the SOCS3 promoter of the 5′UTR domain (M and U indicate methylated and unmethylated domain specific primer sets) and (C) densitometric data from two donors. (D–F) Relative levels of SOCS3, STAT3, and phosphorylated STAT3 as determined by immunoblotting and densitometry (n=5). (G–I) Effect of 5-aza-2′-deoxycytidine (+/−DAC) pretreatment after addition of IL-6 (solid columns) or not (open columns). (G) A representative gel image (inset) and relative densitometric values (n=3) for SOCS3 promoter methylation (G) and relative densitometric values of immunoblots for SOCS3 (H, n=4) and phosphorylated STAT3 (I. n=5). Values are mean ± SEM of values. Significant differences: * P<0.05, ** P<0.01, *** P<0.001.
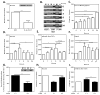
FIG 6. Reversibility of IL-6 effects
Data indicate days after withdrawal of IL-6 from SCF/IL-6-conditioned HuMC cultures. (A) Degranulation with SA, 10 ng/ml (n=2), (B) methylation of SOCS3 promoter (5′UTR domain, boxed region) in a representative culture or (C) densitometric data from all donors (n=3), and (D–F) expression of SOCS3 (n=5), STAT3 (n=6), and phosphorylated STAT3 (n=7) as indicated by representative immunoblots and densitometric values for all donors. Values are relative mean values ± SEM (day zero equals 100). * P<0.05, **P<0.01, ***P<0.001.
Similar articles
- Effect of lipopolysaccharide (LPS) and peptidoglycan (PGN) on human mast cell numbers, cytokine production, and protease composition.
Kirshenbaum AS, Swindle E, Kulka M, Wu Y, Metcalfe DD. Kirshenbaum AS, et al. BMC Immunol. 2008 Aug 7;9:45. doi: 10.1186/1471-2172-9-45. BMC Immunol. 2008. PMID: 18687131 Free PMC article. - Regulatory effect of calcineurin inhibitor, tacrolimus, on IL-6/sIL-6R-mediated RANKL expression through JAK2-STAT3-SOCS3 signaling pathway in fibroblast-like synoviocytes.
Choe JY, Park KY, Park SH, Lee SI, Kim SK. Choe JY, et al. Arthritis Res Ther. 2013 Feb 13;15(1):R26. doi: 10.1186/ar4162. Arthritis Res Ther. 2013. PMID: 23406906 Free PMC article. - An optimized protocol for the generation and functional analysis of human mast cells from CD34+ enriched cell populations.
Yin Y, Bai Y, Olivera A, Desai A, Metcalfe DD. Yin Y, et al. J Immunol Methods. 2017 Sep;448:105-111. doi: 10.1016/j.jim.2017.06.003. Epub 2017 Jun 16. J Immunol Methods. 2017. PMID: 28629733 Free PMC article. - Mast cells signal their importance in health and disease.
Olivera A, Beaven MA, Metcalfe DD. Olivera A, et al. J Allergy Clin Immunol. 2018 Aug;142(2):381-393. doi: 10.1016/j.jaci.2018.01.034. Epub 2018 Feb 15. J Allergy Clin Immunol. 2018. PMID: 29454835 Review. - Interleukin-6: A Masterplayer in the Cytokine Network.
Uciechowski P, Dempke WCM. Uciechowski P, et al. Oncology. 2020;98(3):131-137. doi: 10.1159/000505099. Epub 2020 Jan 20. Oncology. 2020. PMID: 31958792 Review.
Cited by
- Mast cell-T cell axis alters development of colitis-dependent and colitis-independent colorectal tumours: potential for therapeutically targeting via mast cell inhibition.
Sakita JY, Elias-Oliveira J, Carlos D, de Souza Santos E, Almeida LY, Malta TM, Brunaldi MO, Albuquerque S, Araújo Silva CL, Andrade MV, Bonato VLD, Garcia SB, Cunha FQ, Cebinelli GCM, Martins RB, Matthews J, Colli L, Martin FL, Uyemura SA, Kannen V. Sakita JY, et al. J Immunother Cancer. 2022 Oct;10(10):e004653. doi: 10.1136/jitc-2022-004653. J Immunother Cancer. 2022. PMID: 36220303 Free PMC article. - The role of human mast cells in allergy and asthma.
Banafea GH, Bakhashab S, Alshaibi HF, Natesan Pushparaj P, Rasool M. Banafea GH, et al. Bioengineered. 2022 Mar;13(3):7049-7064. doi: 10.1080/21655979.2022.2044278. Bioengineered. 2022. PMID: 35266441 Free PMC article. Review. - The Role of Crosstalk of Immune Cells in Pathogenesis of Chronic Spontaneous Urticaria.
Zhou B, Li J, Liu R, Zhu L, Peng C. Zhou B, et al. Front Immunol. 2022 May 31;13:879754. doi: 10.3389/fimmu.2022.879754. eCollection 2022. Front Immunol. 2022. PMID: 35711438 Free PMC article. Review. - Genetic evidence for the role of transforming growth factor-β in atopic phenotypes.
Weissler KA, Frischmeyer-Guerrerio PA. Weissler KA, et al. Curr Opin Immunol. 2019 Oct;60:54-62. doi: 10.1016/j.coi.2019.05.002. Epub 2019 Jun 1. Curr Opin Immunol. 2019. PMID: 31163387 Free PMC article. Review. - The Role of Mast Cells in Bone Metabolism and Bone Disorders.
Ragipoglu D, Dudeck A, Haffner-Luntzer M, Voss M, Kroner J, Ignatius A, Fischer V. Ragipoglu D, et al. Front Immunol. 2020 Feb 7;11:163. doi: 10.3389/fimmu.2020.00163. eCollection 2020. Front Immunol. 2020. PMID: 32117297 Free PMC article. Review.
References
- Hirano T. Interleukin 6 and its receptor: Ten years later. Intern Rev Immunol. 1998;16:249–84. - PubMed
- Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–59. - PubMed
- Brockow K, Akin C, Huber M, Metcalfe DD. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin Immunol. 2005;115:216–23. - PubMed
- Mayado A, Teodosio C, Garcia-Montero AC, Matito A, Rodriguez-Caballero A, Morgado JM, et al. Increased IL6 plasma levels in indolent systemic mastocytosis patients are associated with high risk of disease progression. Leukemia. 2015:176. - PubMed
- Fujii K, Konishi K, Kanno Y, Ohgou N. Acute urticaria with elevated circulating interleukin-6 is resistant to anti-histamine treatment. J Dermatol. 2001;28:248–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials