Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation - PubMed (original) (raw)
Ginkgolide B protects human umbilical vein endothelial cells against xenobiotic injuries via PXR activation
Tao Zhou et al. Acta Pharmacol Sin. 2016 Feb.
Abstract
Aim: Pregnane X receptor (PXR) is a nuclear receptor that regulates a number of genes encoding drug metabolism enzymes and transporters and plays a key role in xeno- and endobiotic detoxification. Ginkgolide B has shown to increase the activity of PXR. Here we examined whether ginkgolide B activated PXR and attenuated xenobiotic-induced injuries in endothelial cells.
Methods: Human umbilical vein endothelial cells (HUVECs) were treated with ginkgolide B. The expression of PXR, CYP3A4, MDR1, VCAM-1, E-selectin and caspase-3 were quantified with qRT-PCR and Western blot analysis. Cell apoptosis was analyzed with flow cytometry. Fluorescently labeled human acute monocytic leukemia cells (THP-1 cells) were used to examine cell adhesion.
Results: Ginkgolide B (30-300 μmol/L) did not change the mRNA and protein levels of PXR in the cells, but dose-dependently increased nuclear translocation of PXR protein. Ginkgolide B increased the expression of CYP3A4 and MDR1 in the cells, which was partially reversed by pretreatment with the selective PXR signaling antagonist sulforaphane, or transfection with PXR siRNA. Functionally, ginkgolide B dose-dependently attenuated doxorubicin- or staurosporine-induced apoptosis, which was reversed by transfection with PXR siRNA. Moreover, ginkgolide B suppressed TNF-α-induced THP-1 cell adhesion and TNF-α-induced expression of vascular adhesion molecule 1 (VCAM-1) and E-selectin in the cells, which was also reversed by transfection with PXR siRNA.
Conclusion: Ginkgolide B exerts anti-apoptotic and anti-inflammatory effects on endothelial cells via PXR activation, suggesting that a PXR-mediated endothelial detoxification program may be important for protecting endothelial cells from xeno- and endobiotic-induced injuries.
Figures
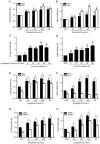
Figure 1
Ginkgolide B regulates PXR activity in HUVECs. HUVECs were treated with ginkgolide B (30, 150, and 300 μmol/L) for 24 h, and RIF treatment was a positive control. (A) PXR mRNA was measured by qRT-PCR. (B) Protein expression was measured with Western blot. (C) The nuclear and cytosolic PXR protein expression levels were quantified by Western blot analysis. Histone and β-actin were internal controls for nuclear and cytosolic protein, respectively. Data are shown as mean±SEM. n_=3. a_P_>0.05, b_P<0.05 vs control group.
Figure 2
Ginkgolide B upregulates CYP3A4 and MDR1 mRNA expression via PXR activation. (A, B) Cells were treated with ginkgolide B (30, 150, or 300 μmol/L) for 24 and 48 h; RIF treatment served as a positive control. (C, D) Cells were treated with ginkgolide B (150 μmol/L) for 6 to 48 h. (E, F) HUVECs were pretreated with SFN (10 μmol/L) for 24 h and then treated with ginkgolide B for 24 h. (G, H) Cells were transfected with PXR siRNA and exposed to ginkgolide B for 48 h later. The CYP3A4 and MDR1 mRNA expression levels were analyzed by qRT-PCR. Data are expressed as mean±SEM. n_=3–5. a_P_>0.05, b_P<0.05, c_P_<0.01 vs control group. e_P_<0.05, f_P_<0.01 vs SFN group. h_P_<0.05 vs PXR siRNA group.
Figure 3
Ginkgolide B attenuates HUVEC apoptosis. (A) HUVECs were pretreated with ginkgolide B (30, 150, or 300 μmol/L) for 24 h in the presence or absence of PXR siRNA and then treated with 500 nmol/L of ST for an additional 6 h. Positive control cells were treated with ST alone for 6 h. (B) Cells were pretreated with ginkgolide B for 24 h and then treated with 5 μmol/L of DOX for an additional 24 h. Positive control cells were treated with DOX alone for 24 h. (C) Cells were transfected with PXR siRNA and exposed to ginkgolide B for 24 h 48 h later; cells were treated with DOX for an additional 24 h. Apoptosis was assessed with flow cytometry for annexin V-FITC. (D) The intracellular DOX concentration was measured based on the fluorescence intensity in HUVECs pretreated with ginkgolide B in the presence or absence of PXR siRNA before exposure to DOX using flow cytometry. (E, F) Caspase 3 cleavage was measured with Western blotting. Data shown are mean±SEM. n_=3–6. b_P<0.05, c_P_<0.01 vs_ control group. d_P_>0.05, e_P<0.05 vs MOCK group. h_P_< 0.05 vs PXR siRNA group.
Figure 4
Ginkgolide B suppresses pro-inflammatory response in HUVECs. HUVECs pretreated with ginkgolide B (30, 150, or 300 μmol/L) for 24 h in the absence (A, B) or presence (C, D) of PXR siRNA before exposure to TNF-α (10 ng/mL). HUVECs were then incubated with fluorescently labeled THP-1 cells (5×105 cells/mL) for 30 min. THP-1 adhesions were counted under a fluorescent microscope. Bar=10 μm. Data shown are as mean±SEM. n_=8. c_P<0.01 vs_ control. d_P_>0.05, e_P<0.05 vs MOCK group. AM: under calcein-AM fluorescence; Normal: under normal light.
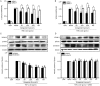
Figure 5
Ginkgolide B suppresses pro-inflammatory response factors in HUVECs. (A–D) HUVECs pretreated with ginkgolide B (30, 150, or 300 μmol/L) for 24 h in the presence or absence of PXR siRNA before exposure to TNF-α (10 ng/mL). (A, B) VCAM-1 and E-selectin mRNA were measured by qRT-PCR. (C, D) VCAM-1 and E-selectin protein expression was measured with Western blot. Data shown are mean±SEM. n_=3. c_P<0.01 vs_ control. d_P_>0.05, e_P<0.05 vs MOCK group. h_P_<0.05 vs PXR siRNA group. MOCK' group: MOCK+siRNA.
References
- 3Nishimura M, Naito S, Yokoi T. Tissue-specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metab Pharmacokin 2004; 19: 135–49. - PubMed
- 4Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocrine Rev 2002; 23: 687–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous