Ligand Activation of ERRα by Cholesterol Mediates Statin and Bisphosphonate Effects - PubMed (original) (raw)
Ligand Activation of ERRα by Cholesterol Mediates Statin and Bisphosphonate Effects
Wei Wei et al. Cell Metab. 2016.
Abstract
Nuclear receptors (NRs) are key regulators of gene expression and physiology. Nearly half of all human NRs lack endogenous ligands including estrogen-related receptor α (ERRα). ERRα has important roles in cancer, metabolism, and skeletal homeostasis. Affinity chromatography of tissue lipidomes with the ERRα ligand-binding domain (LBD) and subsequent transcriptional assays identified cholesterol as an endogenous ERRα agonist. Perturbation of cholesterol biosynthesis or inhibition of ERRα revealed the interdependence of cholesterol and ERRα. In bone, the effects of cholesterol, statin, and bisphosphonate on osteoclastogenesis require ERRα; and consequently, cholesterol-induced bone loss or bisphosphonate osteoprotection is lost in ERRα knockout mice. Furthermore, statin induction of muscle toxicity and cholesterol suppression of macrophage cytokine secretion are impaired by loss or inhibition of ERRα. These findings reveal a key step in ERRα regulation and explain the actions of two highly prescribed drugs, statins and bisphosphonates.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no financial conflict of interest.
Figures
Figure 1. The ERRα-LBD selectively enriches cholesterol from the lipidome
(A) A schematic diagram of the workflow for the identification of HIS-ERRα-LBD binders. HIS-ERRα-LBD is immobilized on a solid support and incubated with lipids from brain or kidney. After washing away unbound lipids, the protein complex is eluted, and eluent is analyzed by LC-MS. Lipids that bind HIS-ERRα-LBD are only present in the ERRα sample, but not in the no protein (resin only) control. (B) HIS-ERRα-LBD was incubated with a pool of lipids from brain, and bound lipids were analyzed by untargeted LC-MS. Cholesterol was the only lipid that was strongly and consistently enriched. (C) Cholesterol binding is also demonstrated by a targeted MS/MS method designed for sensitive and specific identification of sterol HIS-ERRα-LBD binding. A representative LC-MS chromatogram is shown. (D) Cholesterol binding could be blocked by the ERRα synthetic antagonist diethylstilbestrol (DES). In the absence of DES, HIS-ERRα-LBD strongly enriched cholesterol from a pool of brain sterols relative to a resin only sample. When DES was added to the sterol mixture, HIS-ERRα-LBD could no longer enrich cholesterol. Error bars, SD. See also Figure S1, Figure S2 and Table S1.
Figure 2. Characterization of cholesterol binding to the ERRα-LBD
(A) Molecular docking simulations suggested that cholesterol bound to ERRα in the ligand binding pocket with the hydroxyl group facing into the ligand binding pocket. In this model, cholesterol makes important interactions with three residues: E235, F232, and L228 (highlighted in blue). (B) Fluorescent cholesterol derivatives demonstrated that cholesterol bound with its hydroxyl group facing into the ligand binding pocket. Labeling at the alkyl but not hydroxyl group with an NDB fluorophore permitted ERRα-cholesterol binding. (C) Competition fluorescence polarization assay with increasing amount of cholesterol and estradiol indicates that only cholesterol was able to compete with 25-NBD-cholesterol in binding to ERRα-LBD. (D) Cholesterol binding quenches the intrinsic tryptophan fluorescence intensity of ERRα-LBD, while estradiol, which is known to not bind ERRα, has no effect on ERRα tryptophan fluorescence. (E) A E235A, F232A, L228A ERRα triple mutant had impaired cholesterol binding ability relative to wild type ERRα. Error bars, SD. See also Figure S3.
Figure 3. Cholesterol increases ERRα transcriptional activity
ERRα transcriptional activity was analyzed using transient transfection and reporter assays. (A) Cholesterol depletion by lovastatin and/or the cholesterol binder hydroxypropyl-beta-cyclodextrin (HPCD) results in lower ERRα transcriptional activity. Results are shown as fold induction by ERRα+PGC1α transfection compared to vector (vec) transfection control (n=6). * indicates HPCD effect; + indicates lovastain effect. (B) The addition of cholesterol (blue bars) back to cholesterol-depleted cells dose-dependently rescues ERRα activity; whereas XCT790 (brown bars), a known ERRα antagonist and thus a control for this assay, further suppressed ERRα activity dose-dependently (n=6). CV-1 cells were transfected with expression vectors for ERRα and PGC1α co-activator as well as ERRE-luc and CMV-βgal reporters. For cholesterol depletion, the cells were treated with lovastatin at indicated concentration and HPCD (10mM) for 4hrs in DMEM containing 10% NCLPPS (newborn calf lipoprotein poor serum), and then with lovastatin in DMEM containing 10% NCLPPS for another 20hrs. For cholesterol additions to cells, cholesterol-depleted cells were treated with cholesterol or vehicle during the 20hrs of lovastatin treatment, or with XCT790 as a control. Error bars, SD. See also Figure S4.
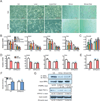
Figure 4. ERRα mediates statin-induced muscle toxicity
(A–B) Effects of statins, cholesterol and XCT790 on C2C12 myotube differentiation. Lovastatin (Lova), simvastatin (Simva), cholesterol and XCT790 were added at 10µM. (A) Representative images of C2C12 differentiation cultures on day 5. Scale bar, 25µm. (B) mRNA expression of myotube differentiation markers on day 5 (n=3). (C) mRNA expression of the muscle atrophy gene atrogin-1 (Fbxo32) on day 5 (n=3). (D–F) Statin-induced muscle toxicity is abolished in ERRαKO mice. ERRαKO mice or WT controls (6 month old, females, n=6) were treated with simvastatin (Simva) for 2 weeks at 20mg/kg/day. (D) mRNA expression of myotube differentiation markers in quadriceps muscle (n=6). (E) mRNA expression of the muscle atrophy gene atrogin-1 (Fbxo32) in quadriceps muscle (n=6). (F) Serum muscle damage marker creatine kinase (CK, n=6). Black * and n.s. compare statin with vehicle (Veh) control; blue + and n.s. compare ERRαKO with WT control. (G) Co-immunoprecipitation analysis of the effects of statin and cholesterol on ERRα and PGC1α interaction. C2C12 myotube differentiation cultures were treated with vehicle, simvastatin or simvastatin+cholesterol at 10µM for 24 hr. Average results for the IP/Input ratio from 3 independent experiments are shown. Error bars, SD. See also Figure S5 and S7.
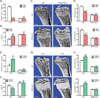
Figure 5. ERRα mediates cholesterol stimulation of osteoclastogenesis ex vivo
(A–B) Cholesterol promotes whereas statin and bisphosphonate inhibit osteoclastogenesis in WT but not ERRαKO bone marrow osteoclast differentiation cultures. (A) Representative images of TRAP-stained differentiation cultures showing the number and size of mature osteoclasts. Mature osteoclasts were identified as multinucleated (>3 nuclei) TRAP+ (purple) cells. Scale bar, 25µm. Number of osteoclast/well is shown as insets; black * or n.s. compare cholesterol vs. veh, red * or n.s. compare lovastatin vs. control, green * or n.s. compare zoledronate vs. control. (B) Quantification of the mRNA expression of ERRα target genes and osteoclast differentiation markers. P-values compare each treatment condition with vehicle control in the same genotype. (C) Cholesterol induction of ERRα target genes and osteoclast differentiation markers in WT bone marrow osteoclast differentiation cultures was abolished by the treatment of an ERRα antagonist XCT790. Chol, cholesterol (10µM); lova, lovastatin (10µM); zole, zoledronate (20µM); ctrl, control; XCT, XCT790 (10µM). (D) Co-immunoprecipitation analysis of the effects of statin and cholesterol on ERRα and PGC1β interaction. Bone marrow osteoclast differentiation cultures were treated with RANKL for 6 days in the presence of lovastatin (10nM), cholesterol (2µM) or vehicle control. Representative results from three independent experiments are shown. (E) Cholesterol inhibits whereas statin and bisphosphonate enhance cxcl9 and cxcl10 expression in WT but not ERRαKO macrophages. Macrophages were differentiated from bone marrow cells of WT or ERRαKO mice with 20ng/ml MCSF for 8 days, in the presence of compound or vehicle, and then treated with 200µg/ml LPS for 6hr at the end. P-values compare each treatment condition with vehicle control in the same genotype. (F) Cholesterol suppression of cxcl9 and cxcl10 in WT macrophages was abolished by XCT790. Chol, cholesterol (10µM); lova, lovastatin (10µM); zole, zoledronate (20µM); ctrl, control; XCT, XCT790 (10µM). Error bars, SD. See also Figure S5 and S6.
Figure 6. ERRα mediates cholesterol stimulation of bone resorption in vivo
(A–D) Osteoprotective effects of zoledronate were abolished in ERRαKO mice. WT or ERRαKO mice (6 week old male, n=4) were treated with a single i.v. injection of zoledronate at 0.54 mg/kg or PBS vehicle control and analyzed 4 weeks later. (A) Serum levels of the bone resorption marker CTX-1. (B–C) MicroCT analysis of tibiae. (B) Trabecular bone volume/tissue volume ratio (BV/TV). (C) Representative CT images of the entire proximal tibia (scale bar, 1mm). (D) Bone histomorphometry analysis of osteoclast surface/bone surface (Oc.S/BS) (top) and osteoclast number/bone area (Oc.N/B.Ar) (bottom). (E–H) Bone loss induced by high cholesterol diet (HCD) feeding was abolished in ERRαKO mice. WT or ERRαKO mice (7 week old female, n=4) were fed with a HCD or chow control diet for 4 weeks. (E) Serum levels of the bone resorption marker CTX-1. (F–G) MicroCT analysis of tibiae. (F) Trabecular BV/TV. (G) Representative CT images of the entire proximal tibia (scale bar, 1mm). (H) Bone histomorphometry analysis of Oc.S/BS (top) and Oc.N/B.Ar (bottom). Error bars, SD. See also Figure S7.
Figure 7. A schematic diagram of how ERRα mediates the effects of cholesterol, statins and bisphosphonates
Cholesterol serves as a natural ERRα agonist to recruit coactivators PGC1α/β and increase ERRα transcriptional activity, thereby promoting osteoclastogenesis and myogenesis but inhibiting macrophage cytokine production. A reduction in cholesterol synthesis by statins or bisphosphonates decreases ERRα transcriptional activity, thereby mediating the statin-induced muscle toxicity and bisphosphonate suppression of bone resorption.
Similar articles
- The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on HMG-CoA reductase activity.
Staal A, Frith JC, French MH, Swartz J, Güngör T, Harrity TW, Tamasi J, Rogers MJ, Feyen JH. Staal A, et al. J Bone Miner Res. 2003 Jan;18(1):88-96. doi: 10.1359/jbmr.2003.18.1.88. J Bone Miner Res. 2003. PMID: 12510809 - Computational insights into the interaction mechanisms of estrogen-related receptor alpha with endogenous ligand cholesterol.
Li D, Cai Y, Teng D, Li W, Tang Y, Liu G. Li D, et al. Chem Biol Drug Des. 2019 Jul;94(1):1316-1329. doi: 10.1111/cbdd.13506. Epub 2019 Mar 20. Chem Biol Drug Des. 2019. PMID: 30811808 - PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism.
Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP. Wende AR, et al. Mol Cell Biol. 2005 Dec;25(24):10684-94. doi: 10.1128/MCB.25.24.10684-10694.2005. Mol Cell Biol. 2005. PMID: 16314495 Free PMC article. - Mevalonate-suppressive dietary isoprenoids for bone health.
Mo H, Yeganehjoo H, Shah A, Mo WK, Soelaiman IN, Shen CL. Mo H, et al. J Nutr Biochem. 2012 Dec;23(12):1543-51. doi: 10.1016/j.jnutbio.2012.07.007. Epub 2012 Sep 13. J Nutr Biochem. 2012. PMID: 22981371 Review. - Cholesterol as an Endogenous ERRα Agonist: A New Perspective to Cancer Treatment.
Casaburi I, Chimento A, De Luca A, Nocito M, Sculco S, Avena P, Trotta F, Rago V, Sirianni R, Pezzi V. Casaburi I, et al. Front Endocrinol (Lausanne). 2018 Sep 11;9:525. doi: 10.3389/fendo.2018.00525. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30254608 Free PMC article. Review.
Cited by
- The bacterial quorum sensing signal 2'-aminoacetophenone rewires immune cell bioenergetics through the Ppargc1a/Esrra axis to mediate tolerance to infection.
Chakraborty A, Bandyopadhaya A, Singh VK, Kovacic F, Cha S, Oldham WM, Tzika AA, Rahme LG. Chakraborty A, et al. Elife. 2024 Sep 13;13:RP97568. doi: 10.7554/eLife.97568. Elife. 2024. PMID: 39269443 Free PMC article. - Crosstalk between Lipid Metabolism and Bone Homeostasis: Exploring Intricate Signaling Relationships.
Xiao H, Li W, Qin Y, Lin Z, Qian C, Wu M, Xia Y, Bai J, Geng D. Xiao H, et al. Research (Wash D C). 2024 Aug 20;7:0447. doi: 10.34133/research.0447. eCollection 2024. Research (Wash D C). 2024. PMID: 39165638 Free PMC article. - LIFR regulates cholesterol-driven bidirectional hepatocyte-neutrophil cross-talk to promote liver regeneration.
Deng Y, Zhao Z, Sheldon M, Zhao Y, Teng H, Martinez C, Zhang J, Lin C, Sun Y, Yao F, Curran MA, Zhu H, Ma L. Deng Y, et al. Nat Metab. 2024 Sep;6(9):1756-1774. doi: 10.1038/s42255-024-01110-y. Epub 2024 Aug 15. Nat Metab. 2024. PMID: 39147934 - Targeting adipocyte ESRRA promotes osteogenesis and vascular formation in adipocyte-rich bone marrow.
Huang T, Lu Z, Wang Z, Cheng L, Gao L, Gao J, Zhang N, Geng CA, Zhao X, Wang H, Wong CW, Yeung KWK, Pan H, Lu WW, Guan M. Huang T, et al. Nat Commun. 2024 May 4;15(1):3769. doi: 10.1038/s41467-024-48255-8. Nat Commun. 2024. PMID: 38704393 Free PMC article. - Impact of De Novo Cholesterol Biosynthesis on the Initiation and Progression of Breast Cancer.
Coradini D. Coradini D. Biomolecules. 2024 Jan 3;14(1):64. doi: 10.3390/biom14010064. Biomolecules. 2024. PMID: 38254664 Free PMC article. Review.
References
- Andersson HC, Kratz L, Kelley R. Desmosterolosis presenting with multiple congenital anomalies and profound developmental delay. American journal of medical genetics. 2002;113:315–319. - PubMed
- Ascenzi P, Bocedi A, Marino M. Structure-function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med. 2006;27:299–402. - PubMed
- Ayers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry. 2007;46:6744–6752. - PubMed
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases