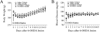Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats - PubMed (original) (raw)
Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats
Y Pang et al. Neuroscience. 2016.
Abstract
Protection of substantia nigra (SN) dopaminergic (DA) neurons by neurotrophic factors (NTFs) is one of the promising strategies in Parkinson's disease (PD) therapy. A major clinical challenge for NTF-based therapy is that NTFs need to be delivered into the brain via invasive means, which often shows limited delivery efficiency. The nose to brain pathway is a non-invasive brain drug delivery approach developed in recent years. Of particular interest is the finding that intranasal insulin improves cognitive functions in Alzheimer's patients. In vitro, insulin has been shown to protect neurons against various insults. Therefore, the current study was designed to test whether intranasal insulin could afford neuroprotection in the 6-hydroxydopamine (6-OHDA)-based rat PD model. 6-OHDA was injected into the right side of striatum to induce a progressive DA neuronal lesion in the ipsilateral SN pars compact (SNc). Recombinant human insulin was applied intranasally to rats starting from 24h post lesion, once per day, for 2 weeks. A battery of motor behavioral tests was conducted on day 8 and 15. The number of DA neurons in the SNc was estimated by stereological counting. Our results showed that 6-OHDA injection led to significant motor deficits and 53% of DA neuron loss in the ipsilateral side of injection. Treatment with insulin significantly ameliorated 6-OHDA-induced motor impairments, as shown by improved locomotor activity, tapered/ledged beam-walking performance, vibrissa-elicited forelimb-placing, initial steps, as well as methamphetamine-induced rotational behavior. Consistent with behavioral improvements, insulin treatment provided a potent protection of DA neurons in the SNc against 6-OHDA neurotoxicity, as shown by a 74.8% increase in tyrosine hydroxylase (TH)-positive neurons compared to the vehicle group. Intranasal insulin treatment did not affect body weight and blood glucose levels. In conclusion, our study showed that intranasal insulin provided strong neuroprotection in the 6-OHDA rat PD model, suggesting that insulin signaling may be a novel therapeutic target in broad neurodegenerative disorders.
Keywords: Parkinson’s disease; dopaminergic neurons; insulin; neurotrophin; striatum; substantia nigra.
Copyright © 2016 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
Fig. 1
Intranasal insulin significantly ameliorates locomotor and exposure rearing impairment in rats subjected to unilaterally 6-OHDA lesion. 6-OHDA treatment significantly reduced both crossing distance (A: Open field test) and rearing events (B: Exposure rearing response) on day 15. Treatment with intranasal insulin significantly ameliorated deficits in both locomotion and rearing events in 6-OHDA-lesion rats. Data were analyzed by two-way repeated measures ANOVA followed by Student-Newman-Keuls test. N=16 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group, on the same post-treatment day. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group on the same post-treatment day.
Fig. 2
Intranasal insulin significantly improved motor performance in tapered/ledged beam walking tests in 6-OHDA lesion rats. At both day 8 and 15, 6-OHDA lesioned rats showed significantly increased incidence of slip steps and response latency as compared to the control. Treatment with intranasal insulin completely reversed 6-OHDA-induced motor impairment. Data were analyzed by two-way repeated measures ANOVA followed by Student-Newman-Keuls test. N=16 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group, on the same post-treatment day. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group on the same post-treatment day.
Fig 3
Intranasal insulin significantly improved forelimb sensorimotor deficits. A marked decrease in the successful rate of forelimb placing in response to vibrissa stimulation was observed in the 6-OHDA-lesioned rats on day 8 and 15 (A). A significant, progressive increase of the response latency to initiate steps was also observed in 6-OHDA-lesioned rats (B). Daily treatment with intranasal insulin significantly increased the success rate of forelimb-placing (A), and reduced the response latency to initiate steps (B). N=16 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group, on the same post-treatment day. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group on the same post-treatment day.
Fig. 4
Intranasal insulin significantly reduced METH-induced rotational behaviors in 6-OHDA-lesioned rats. The METH-induced rotation was calculated as the net value by subtracting the contralateral rotation from the ipsilateral rotation assessed on day 15 post-lesion. 6-OHDA-lesioned rats showed a markedly increased rotation towards the lesion side, which was significantly reduced by treatment with intranasal insulin. N=8 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group.
Fig. 5
Intranasal insulin treatment protected nigral DA neurons against 6-OHDA neurotoxicity. A: representative micrographs illustrate TH immunostaining in the SN regions. 6-OHDA lesion rats showed a marked loss of TH+ DA neurons in the ipsilateral side, primarily located in the SNc but also noticeable in the SN reticulate and VTA regions. In contrast, TH immunostaining in the contralateral side was not affected. Treatment with insulin protected TH+ neurons in the ipsilateral SNc against 6-OHDA lesion. B: Stereological cell counting to estimate numbers of DA neurons in both side of the SNc. 6-OHDA exposure led to a 53% loss of DA neurons in the ipsilateral SNc, while insulin treatment significantly increased DA neuron survival in the 6-OHDA-exposed animals (P<0.05). N=10 for the saline+vehicle, saline+insulin, and 6-OHDA+vehicle groups; n=9 for the 6-OHDA+insulin group. * P<0.05 vs the right side of the saline+vehicle or saline+insulin group; #p<0.05 vs the right side of the 6-OHDA+vehicle group.
Fig. 6
Intranasal insulin did not affect body weight and blood glucose levels. A: The body weight of all experimental animals decreased in the first few days after the surgery; however, all animals were able to regain body weight to the pre-surgery levels afterward. There were no significant differences among groups. Similarly, blood glucose levels dropped sharply in all animals at 24 h post-surgery, but returned to pre-surgery levels afterwards. There were no significant differences of blood glucose levels among treatment groups. *P<0.05 vs before surgery. N=16 in each group.
Similar articles
- Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents.
Nie S, Xu Y, Chen G, Ma K, Han C, Guo Z, Zhang Z, Ye K, Cao X. Nie S, et al. Neuropharmacology. 2015 Dec;99:448-58. doi: 10.1016/j.neuropharm.2015.08.016. Epub 2015 Aug 14. Neuropharmacology. 2015. PMID: 26282118 - The Kv7/KCNQ channel blocker XE991 protects nigral dopaminergic neurons in the 6-hydroxydopamine rat model of Parkinson's disease.
Liu H, Jia L, Chen X, Shi L, Xie J. Liu H, et al. Brain Res Bull. 2018 Mar;137:132-139. doi: 10.1016/j.brainresbull.2017.11.011. Epub 2017 Nov 22. Brain Res Bull. 2018. PMID: 29174294 - RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson's Disease.
Ahlers-Dannen KE, Spicer MM, Fisher RA. Ahlers-Dannen KE, et al. Mol Pharmacol. 2020 Dec;98(6):730-738. doi: 10.1124/mol.119.118836. Epub 2020 Feb 3. Mol Pharmacol. 2020. PMID: 32015009 Free PMC article. Review. - Neonatal 6-hydroxydopamine lesioning of rats and dopaminergic neurotoxicity: proposed animal model of Parkinson's disease.
Kostrzewa RM. Kostrzewa RM. J Neural Transm (Vienna). 2022 Jun;129(5-6):445-461. doi: 10.1007/s00702-022-02479-4. Epub 2022 Mar 12. J Neural Transm (Vienna). 2022. PMID: 35279767 Review.
Cited by
- Electro-acupuncture-induced neuroprotection is associated with activation of the IGF-1/PI3K/Akt pathway following adjacent dorsal root ganglionectomies in rats.
Hu T, Lu MN, Chen B, Tong J, Mao R, Li SS, Dai P, Tan YX, Xiyang YB. Hu T, et al. Int J Mol Med. 2019 Feb;43(2):807-820. doi: 10.3892/ijmm.2018.4035. Epub 2018 Dec 18. Int J Mol Med. 2019. PMID: 30569108 Free PMC article. - Frailty and Parkinson's disease: the role of diabetes mellitus.
Komici K, Pansini A, Bencivenga L, Rengo G, Pagano G, Guerra G. Komici K, et al. Front Med (Lausanne). 2024 May 30;11:1377975. doi: 10.3389/fmed.2024.1377975. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38882667 Free PMC article. Review. - Early Postnatal Neuroinflammation Produces Key Features of Diffuse Brain White Matter Injury in Rats.
Waddell J, Lin S, Carter K, Truong T, Hebert M, Ojeda N, Fan LW, Bhatt A, Pang Y. Waddell J, et al. Brain Sci. 2024 Sep 27;14(10):976. doi: 10.3390/brainsci14100976. Brain Sci. 2024. PMID: 39451991 Free PMC article. - Potential mechanisms to modify impaired glucose metabolism in neurodegenerative disorders.
McDonald TS, Lerskiatiphanich T, Woodruff TM, McCombe PA, Lee JD. McDonald TS, et al. J Cereb Blood Flow Metab. 2023 Jan;43(1):26-43. doi: 10.1177/0271678X221135061. Epub 2022 Oct 24. J Cereb Blood Flow Metab. 2023. PMID: 36281012 Free PMC article. Review. - Diabetes and Cognitive Impairment: A Role for Glucotoxicity and Dopaminergic Dysfunction.
Pignalosa FC, Desiderio A, Mirra P, Nigro C, Perruolo G, Ulianich L, Formisano P, Beguinot F, Miele C, Napoli R, Fiory F. Pignalosa FC, et al. Int J Mol Sci. 2021 Nov 16;22(22):12366. doi: 10.3390/ijms222212366. Int J Mol Sci. 2021. PMID: 34830246 Free PMC article. Review.
References
- Beccaria K, Canney M, Goldwirt L, Fernandez C, Piquet J, Perier MC, Lafon C, Chapelon JY, Carpentier A. Ultrasound-induced opening of the blood-brain barrier to enhance temozolomide and irinotecan delivery: an experimental study in rabbits. J Neurosurg. 2015;13:1–9. - PubMed
- Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104:29–45. - PubMed
- Bender TS, Migliore MM, Campbell RB, John Gatley S, Waszczak BL. Intranasal administration of glial-derived neurotrophic factor (GDNF) rapidly and significantly increases whole-brain GDNF level in rats. Neuroscience. 2015;303:569–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous