The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases - PubMed (original) (raw)
Review
The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases
Deena Khan et al. Front Immunol. 2016.
Abstract
Analogous to other physiological systems, the immune system also demonstrates remarkable sex differences. Although the reasons for sex differences in immune responses are not precisely understood, it potentially involves differences in sex hormones (estrogens, androgens, and differential sex hormone receptor-mediated events), X-chromosomes, microbiome, epigenetics among others. Overall, females tend to have more responsive and robust immune system compared to their male counterparts. It is therefore not surprising that females respond more aggressively to self-antigens and are more susceptible to autoimmune diseases. Female hormone (estrogen or 17β-estradiol) can potentially act on all cellular subsets of the immune system through estrogen receptor-dependent and -independent mechanisms. This minireview highlights differential expression of estrogen receptors on immune cells, major estrogen-mediated signaling pathways, and their effect on immune cells. Since estrogen has varied effects in female-predominant autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus, we will mechanistically postulate the potential differential role of estrogen in these chronic debilitating diseases.
Keywords: MS; SLE; autoimmune; estrogen; immune cell; signaling.
Figures
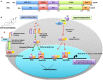
Figure 1
Structural description and percent sequence homology of human ERα and ERβ and schematic representation of estrogen receptor ligand-dependent and ligand-independent signaling. (A) shows comparison of human estrogen receptor α (595 aa) and a shorter estrogen receptor β (530 aa). These receptors are evolutionarily conserved and have five distinct structural and functional domains: DNA-binding domain (DBD; C domain), hinge domain (D), ligand-binding domain (LBD; E/F domain), and two transcriptional activation function domains AF-1 (in A/B domain) and AF-2 (in F domain). The binding of ligand (estrogens) to E domain results in conformational changes in the receptor (homo/hetero dimerization). The receptor dimer than translocates inside nuclei with the help of D domain. This domain is also important for post-translational modifications of receptor by acetylation, lipophilic moieties, and ubiquitination. The C domain then recognizes and binds to estrogen-response element (ERE) in DNA. AF-2 region interacts with co-regulatory proteins in ligand-dependent pathway. However, AF-1 region is activated in ligand-independent manner (54). (B) ER-mediated signaling occurs in a ligand-dependent (green arrows) and ligand-independent (red arrows). The ligand-dependent pathway is triggered by binding of either endogenous hormone or a synthetic compound to the ligand-binding domain of ERs in the cytosol. Different ligands induce unique conformational changes of ERs, and receptor dimerization (homodimers: ERα:ERα or ERβ:ERβ or heterodimer: ERα:ERβ), which then translocate into nuclei and bind to specific EREs (consisting of a 5-bp palindrome with a 3-bp spacer; GGTCAnnnTGACC) in the regulatory regions of estrogen responsive genes. This is also called “_classical_” signaling pathway. In “_tethered_” signaling pathway, ligand-activated ERs interact with other transcription factor complexes and bind to non-EREs by attaching to other transcription factors and not with ERE directly. In third ligand-dependent “_non-genomic_” pathway, ligand interacts with plasma membrane-bound ERs via palmitoylation on cysteine447, which results in activation of cytoplasmic signaling pathways, such as protein kinase C (PKC). In ligand-independent signaling pathway, there is phosphorylation/activation of ERs by other active signaling cascades in a cell (55). This activation results in both direct ERE and non-ERE dependent genomic actions. Abbreviation: Akt, protein kinase B; AP-1, activator protein 1; ERE, estrogen-response element; ER, estrogen receptor; ERK, extracellular signal-regulated kinase; FoxP3, fork box 3; GRP30, an orphan G-protein coupled receptor 30; iNOS, inducible nitric oxide synthase; PI3K, phosphatidylinositol-4, 5-bisphosphate 3-kinase; P, indicates phosphorylation.
Similar articles
- Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action.
Ansar Ahmed S, Penhale WJ, Talal N. Ansar Ahmed S, et al. Am J Pathol. 1985 Dec;121(3):531-51. Am J Pathol. 1985. PMID: 3907369 Free PMC article. Review. - Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease.
Khan D, Dai R, Ansar Ahmed S. Khan D, et al. Cell Immunol. 2015 Apr;294(2):70-9. doi: 10.1016/j.cellimm.2015.01.004. Epub 2015 Jan 19. Cell Immunol. 2015. PMID: 25619140 Review. - [Sex hormones, immune disorders, and inflammatory rheumatic diseases].
Kaliterna DM, Perković D, Radić M, Krstulović DM, Borić K, Marinović I. Kaliterna DM, et al. Reumatizam. 2014;61(1):17-22. Reumatizam. 2014. PMID: 25509832 Croatian. - Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production.
Edwards M, Dai R, Ahmed SA. Edwards M, et al. Front Immunol. 2018 Mar 8;9:478. doi: 10.3389/fimmu.2018.00478. eCollection 2018. Front Immunol. 2018. PMID: 29662485 Free PMC article. Review. - Lupus: why women?
Greenstein BD. Greenstein BD. J Womens Health Gend Based Med. 2001 Apr;10(3):233-9. doi: 10.1089/152460901300139989. J Womens Health Gend Based Med. 2001. PMID: 11389783 Review.
Cited by
- Progesterone promotes CXCl2-dependent vaginal neutrophil killing by activating cervical resident macrophage-neutrophil crosstalk.
Gómez-Oro C, Latorre MC, Arribas-Poza P, Ibáñez-Escribano A, Baca-Cornejo KR, Gallego-Valle J, López-Escobar N, Mondéjar-Palencia M, Pion M, López-Fernández LA, Mercader E, Pérez-Milán F, Relloso M. Gómez-Oro C, et al. JCI Insight. 2024 Oct 22;9(20):e177899. doi: 10.1172/jci.insight.177899. JCI Insight. 2024. PMID: 39298265 Free PMC article. - The Sex and Age-Associated Infiltration of B Cells May Result in the Dimorphic Behaviors Observed in Papillary Thyroid Carcinomas.
Yan C, He X, Sun J. Yan C, et al. Int J Gen Med. 2024 Jul 15;17:3057-3072. doi: 10.2147/IJGM.S467704. eCollection 2024. Int J Gen Med. 2024. PMID: 39055976 Free PMC article. - COVID-19 pandemic: what about the gonads?
Selek A, Güçlü M, Bolu ŞE. Selek A, et al. Hormones (Athens). 2021 Jun;20(2):259-268. doi: 10.1007/s42000-021-00277-3. Epub 2021 Mar 17. Hormones (Athens). 2021. PMID: 33730355 Free PMC article. Review. - The great imitator with no diagnostic test: pyoderma gangrenosum.
Alonso-León T, Hernández-Ramírez HH, Fonte-Avalos V, Toussaint-Caire S, E Vega-Memije M, Lozano-Platonoff A. Alonso-León T, et al. Int Wound J. 2020 Dec;17(6):1774-1782. doi: 10.1111/iwj.13466. Epub 2020 Aug 11. Int Wound J. 2020. PMID: 32779354 Free PMC article. - The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination-An Analysis of Serological Response and Side Effects.
Di Resta C, Ferrari D, Viganò M, Moro M, Sabetta E, Minerva M, Ambrosio A, Locatelli M, Tomaiuolo R. Di Resta C, et al. Vaccines (Basel). 2021 May 18;9(5):522. doi: 10.3390/vaccines9050522. Vaccines (Basel). 2021. PMID: 34070196 Free PMC article.
References
- Ansar Ahmed S, Karpuzoglu E, Khan D. Effect of sex steroids on innate and adaptive immunity. In: Klein SL, Roberts CW, editors. Sex Hormones and Immunity to Infection. London: Springer; (2010). p. 19–51.
- Khan D, Cowan C, Ansar Ahmed S. Estrogen and signaling in the cells of immune system. Adv Neuroimm Biol (2012) 3(1):73–93.10.3233/NIB-2012-012039 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources