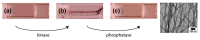The Multiple Faces of Disordered Nucleoporins - PubMed (original) (raw)
Review
The Multiple Faces of Disordered Nucleoporins
Edward A Lemke. J Mol Biol. 2016.
Abstract
An evolutionary advantage of intrinsically disordered proteins (IDPs) is their ability to bind a variety of folded proteins-a paradigm that is central to the nucleocytoplasmic transport mechanism, in which nuclear transport receptors mediate the translocation of various cargo through the nuclear pore complex by binding disordered phenylalanine-glycine-rich nucleoporins (FG-Nups). FG-Nups are highly dynamic, which poses a substantial problem when trying to determine precisely their function using common experimental approaches. FG-Nups have been studied under a variety of conditions, ranging from those that constitute single-molecule measurements to physiological concentrations at which they can form supramolecular structures. In this review, I describe the physicochemical properties of FG-Nups and compare them to those of other disordered systems, including well-studied IDPs. From this comparison, it is apparent that FG-Nups not only share some properties with IDPs in general but also possess unique characteristics that might be key to their central role in the nucleocytoplasmic transport machinery.
Keywords: intrinsically disordered proteins; nucleocytoplasmic transport; phase separation; protein folding and dynamics; protein moonlighting.
Copyright © 2016 The Author. Published by Elsevier Ltd.. All rights reserved.
Figures
Fig. 1
Top view of an EM tomogram of the human NPC, showing an empty channel, according to Ref. [3]. Disordered proteins that fill the channel are not visible to conventional structural biology approaches, and a hole with an approximate diameter of 41 nm is apparent.
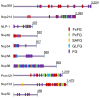
Fig. 2. Sequences of an assortment of disordered human FG-Nup according to UniProt, with different color-coded FG repeats.
Fig. 3
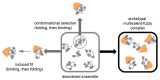
The different binding modes observed for IDP interactions (shown in gray, with valencies indicated by gray square markers) with folded proteins (orange). Due to their dynamics, IDPs populate a disordered ensemble in isolation [109]. In the “conformational selection” binding mode, the folded binding partner can bind to a specific conformation of the ensemble, which can also be a folded state. In the “induced-fit” binding mode, the presence of the binding partner induces folding of the IDP. For FG-domain-NTR interactions, it appears as if the native-state ensemble tends to bind to NTRs so that many conformations can readily engage with the NTR without requiring much time or energy. The result appears to be an archetypal, multivalent fuzzy complex that can form remarkably quickly (reprinted from ref. [46]).
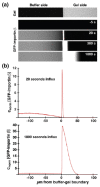
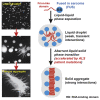
Fig. 4. An experiment to demonstrate how NTRs in solution can rapidly penetrate FG-domain hydrogels.
(a) The interface between an FG-domain hydrogel and a buffer solution. Once a fluorescent NTR (importin-β) was added to the buffer solution outside the gel, the NTR rapidly enriched in the gel boundary. (b) Intensity profiles corresponding to the images in (a), from which it is apparent that a depletion zone exists in at the buffer-gel interface. Adapted from Ref. [71] with permission from Elsevier.
Fig. 5
An FFGEY peptide gel can be switched from a tough hydrogel-like state (a) to a liquid-like state (b) using a kinase and a phosphatase, as demonstrated by Yang et al. [74]. The right-hand panel shows that the gel has a fibrillar ultrastructure as observed by EM, which gives rise to a mechanically stable gel. If the FFGEY peptide is phosphorylated at the Tyr residue by a kinase, the solution is a liquid. If dephosphorylated by action of a phosphatase, an amyloid fiber network forms to a tough gel (a and c). The process is reversible and can be controlled by the addition of a phosphatase or kinase. Adapted with permission from Ref. [74], Copyright (2006) American Chemical Society.
Fig. 6
Cartoon of the pathway by which FUS can undergo various phase transitions to form different assemblies. FUS can undergo liquid–liquid phase separation into droplets. Under certain conditions, these droplets can further age and undergo a liquid–solid phase transition into a fibrillar amyloid-like structure. Reprinted from Ref. [78] with permission from Elsevier.
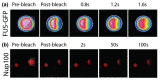
Fig. 7. Half-bleach experiments of phase-separated FUS and FG-Nup100 assemblages (droplets).
(a) Droplets were formed from GFP-labeled FUS, and then only the right-hand half of a droplet was bleached. The fluorescence recovered rapidly (blue to red indicating increasing fluorescence intensity), which points to the droplet being dynamic (liquid-like), and is the result of a liquid-liquid phase separation. (b) A similar half-bleach experiment from a different study for FG-Nup100 (doped with FG-Nup100 labeled with a synthetic fluorophore, shown in red). The droplets appear rigid, which points to the existence of a liquid-gel phase separation. Reprinted from Refs. [78] with permission from Elsevier and [12], respectively.
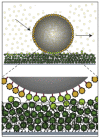
Fig. 8
An experiment in which large beads were coated with NTRs. When these were placed on a layer of densely grafted FG-domains soaked in a solution of NTRs (small spheres in different green and light yellow colors), the beads were able to diffuse on the surface. Adapted with permission from Ref. [91].
Similar articles
- Multifunctionality of F-rich nucleoporins.
Heinß N, Sushkin M, Yu M, Lemke EA. Heinß N, et al. Biochem Soc Trans. 2020 Dec 18;48(6):2603-2614. doi: 10.1042/BST20200357. Biochem Soc Trans. 2020. PMID: 33336681 Free PMC article. Review. - Prevalence and functionality of intrinsic disorder in human FG-nucleoporins.
Lyngdoh DL, Nag N, Uversky VN, Tripathi T. Lyngdoh DL, et al. Int J Biol Macromol. 2021 Apr 1;175:156-170. doi: 10.1016/j.ijbiomac.2021.01.218. Epub 2021 Feb 3. Int J Biol Macromol. 2021. PMID: 33548309 - Floppy but not sloppy: Interaction mechanism of FG-nucleoporins and nuclear transport receptors.
Aramburu IV, Lemke EA. Aramburu IV, et al. Semin Cell Dev Biol. 2017 Aug;68:34-41. doi: 10.1016/j.semcdb.2017.06.026. Epub 2017 Jun 30. Semin Cell Dev Biol. 2017. PMID: 28669824 Free PMC article. Review. - FG nucleoporins feature unique patterns that distinguish them from other IDPs.
Peyro M, Soheilypour M, Nibber VS, Dickson AM, Mofrad MRK. Peyro M, et al. Biophys J. 2021 Aug 17;120(16):3382-3391. doi: 10.1016/j.bpj.2021.06.031. Epub 2021 Jul 6. Biophys J. 2021. PMID: 34237287 Free PMC article. - Deciphering the intrinsically disordered characteristics of the FG-Nups through the lens of polymer physics.
Matsuda A, Mansour A, Mofrad MRK. Matsuda A, et al. Nucleus. 2024 Dec;15(1):2399247. doi: 10.1080/19491034.2024.2399247. Epub 2024 Sep 16. Nucleus. 2024. PMID: 39282864 Free PMC article. Review.
Cited by
- Structural anisotropy results in mechano-directional transport of proteins across nuclear pores.
Panagaki F, Tapia-Rojo R, Zhu T, Milmoe N, Paracuellos P, Board S, Mora M, Walker J, Rostkova E, Stannard A, Infante E, Garcia-Manyes S. Panagaki F, et al. Nat Phys. 2024;20(7):1180-1193. doi: 10.1038/s41567-024-02438-8. Epub 2024 May 13. Nat Phys. 2024. PMID: 39036650 Free PMC article. - Germline NUP98 Variants in Two Siblings with a Rothmund-Thomson-Like Spectrum: Protein Functional Changes Predicted by Molecular Modeling.
Colombo EA, Valiante M, Uggeri M, Orro A, Majore S, Grammatico P, Gentilini D, Finelli P, Gervasini C, D'Ursi P, Larizza L. Colombo EA, et al. Int J Mol Sci. 2023 Feb 16;24(4):4028. doi: 10.3390/ijms24044028. Int J Mol Sci. 2023. PMID: 36835439 Free PMC article. - Benchmarking Molecular Dynamics Force Fields for All-Atom Simulations of Biological Condensates.
Sarthak K, Winogradoff D, Ge Y, Myong S, Aksimentiev A. Sarthak K, et al. J Chem Theory Comput. 2023 Jun 27;19(12):3721-3740. doi: 10.1021/acs.jctc.3c00148. Epub 2023 May 3. J Chem Theory Comput. 2023. PMID: 37134270 Free PMC article. - Kinetic cooperativity resolves bidirectional clogging within the nuclear pore complex.
Zheng T, Zilman A. Zheng T, et al. Biophys J. 2024 May 7;123(9):1085-1097. doi: 10.1016/j.bpj.2024.03.027. Epub 2024 Apr 18. Biophys J. 2024. PMID: 38640928 - HIV-1 capsids enter the FG phase of nuclear pores like a transport receptor.
Fu L, Weiskopf EN, Akkermans O, Swanson NA, Cheng S, Schwartz TU, Görlich D. Fu L, et al. Nature. 2024 Feb;626(8000):843-851. doi: 10.1038/s41586-023-06966-w. Epub 2024 Jan 24. Nature. 2024. PMID: 38267583 Free PMC article.
References
- Bui KH, von Appen A, DiGuilio AL, Ori A, Sparks L, Mackmull MT, Bock T, Hagen W, Andres-Pons A, Glavy JS, Beck M. Integrated structural analysis of the human nuclear pore complex scaffold. Cell. 2013;155:1233–1243. - PubMed
- Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: Hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources