Dynamin-2 is a novel NOS1β interacting protein and negative regulator in the collecting duct - PubMed (original) (raw)
Comparative Study
Dynamin-2 is a novel NOS1β interacting protein and negative regulator in the collecting duct
Kelly A Hyndman et al. Am J Physiol Regul Integr Comp Physiol. 2016.
Abstract
Nitric oxide synthase 1 (NOS1)-derived nitric oxide (NO) production in collecting ducts is critical for maintaining fluid-electrolyte balance. Rat collecting ducts express both the full-length NOS1α and its truncated variant NOS1β, while NOS1β predominates in mouse collecting ducts. We reported that dynamin-2 (DNM2), a protein involved in excising vesicles from the plasma membrane, and NOS1α form a protein-protein interaction that promotes NO production in rat collecting ducts. NOS1β was found to be highly expressed in human renal cortical/medullary samples; hence, we tested the hypothesis that DNM2 is a positive regulator of NOS1β-derived NO production. COS7 and mouse inner medullary collecting duct-3 (mIMCD3) cells were transfected with NOS1β and/or DNM2. Coimmunoprecipitation experiments show that NOS1β and DNM2 formed a protein-protein interaction. DNM2 overexpression decreased nitrite production (index of NO) in both COS7 and mIMCD-3 cells by 50-75%. mIMCD-3 cells treated with a panel of dynamin inhibitors or DNM2 siRNA displayed increased nitrite production. To elucidate the physiological significance of IMCD DNM2/NOS1β regulation in vivo, flox control and CDNOS1 knockout mice were placed on a high-salt diet, and freshly isolated IMCDs were treated acutely with a dynamin inhibitor. Dynamin inhibition increased nitrite production by IMCDs from flox mice. This response was blunted (but not abolished) in collecting duct-specific NOS1 knockout mice, suggesting that DNM2 also negatively regulates NOS3 in the mouse IMCD. We conclude that DNM2 is a novel negative regulator of NO production in mouse collecting ducts. We propose that DNM2 acts as a "break" to prevent excess or potentially toxic NO levels under high-salt conditions.
Keywords: collecting duct; dynamin-2; human; nitric oxide; nitric oxide synthase 1 splice variant.
Copyright © 2016 the American Physiological Society.
Figures
Fig. 1.
Protein expression of NOS1 splice variants in human cortical and medullary homogenates by Western blot analysis. A: five different human samples (see
methods
for demographic information) express both NOS1α (155 kDa) and NOS1β (130 kDa). B: COS7 cells transfected with either NOS1α or NOS1β as a positive control for the COOH-terminal, NOS1-specific antibody.
Fig. 2.
Dynamin-2 (DNM2) and NOS1β interactions in COS7 and mouse inner medullary collecting duct-3 (mIMCD-3) cells. A: COS7 cells were transfected with either NOS1β ± DNM2-GFP. Coimmunoprecipitation experiments were performed by immunoprecipitation (IP) with anti-GFP or mouse IgGs and immunoblot (IB) with anti-NOS1. An interaction is observed in the NOS1β+DNM2 cells only. B: confirmation of the anti-GFP IP with IB with anti-GFP. GFP only expressed in the DNM2-GFP transfected cells. C: mIMCD-3 cells transfected with empty vector or DNM2-GFP. Coimmunoprecipitation experiments were performed by IP with anti-NOS1 and IB with anti-DNM2. NOS1β interacts with both the endogenous DNM2 (100 kDa) and transfected DNM2-GFP (130 kDa), as shown in the bottom panel. D: confirmation of the anti-NOS1 IP with IB with anti-NOS1 (n = 3). L, molecular ladder; V, empty vector transfected cells.
Fig. 3.
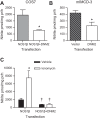
Nitrite production in COS7 and mIMCD-3 cells. A: COS7 cells were transfected with NOS1β ± DNM2 and DNM2 significantly reduced nitrite production (n = 3, *P = 0.007). B: mIMCD-3 cells overexpressing DNM2 produced significantly less nitrite compared with vector control cells (n = 3; *P = 0.04). C: acute stimulation of transfected COS7 cells resulted in a significant increase in nitrite in the NOS1β transfected cells, but no significant effect in the NOS1β+DNM2 cells. *P < 0.05 compared with vehicle. †P < 0.05 compared with corresponding NOS1β vehicle or ionomycin treated cells.
Fig. 4.
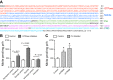
The domains of DNM2 and pharmacological inhibition of DNM2. A: illustration of the different domains in mouse DNM2. PH, plekstrin homology; GED, GTPase effector domain; PRD, proline-rich domain; B: acute pharmacological blockade of the GTPase domain of DNM2 with a panel of inhibitors, resulted in a significant increase in mIMCD-3 nitrite production, compared with their respective controls. C: acute pharmacological inhibition of the PH domain of DNM2 resulted in a significant increase in mIMCD-3 nitrite production, compared with control (P = 0.01). Two-factor ANOVA with Sidak's multiple (6) comparisons were used. *Significant difference compared with control.
Fig. 5.
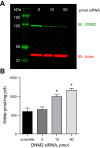
Knockdown of DNM2 by siRNA in mIMCD-3 cells. A: representative image of DNM2 knockdown with 0, 10, or 60 pmol of DNM2 siRNA. B: DNM2 siRNA knockdown results in a significant increase in mIMCD-3 nitrite production compared with scramble siRNA. *P < 0.05 compared with scramble siRNA.
Fig. 6.
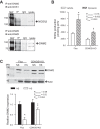
NOS1β and DNM2 form a protein-protein interaction in vivo. Freshly isolated IMCD from flox and collecting duct-specific NOS1 knockout (CDNOS1KO) mice on different salt diets for 7 days. A: coimmunoprecipitation (IP) experiments were performed with anti-DNM2 or rabbit IgGs followed by immunoblot (IB) with anti-NOS1 or anti-DNM2 with IMCD from flox control mice on a HS diet. An interaction is observed between NOS1β and DNM2. B: nitrite production from freshly isolated IMCDs from flox and CDNOS1KO mice on a 7-day high-salt diet (n = 4–10; see
methods
for detailed explanation of sample). Acute 30-min inhibition of DNM2 resulted in a significant increase in nitrite production by flox and tended to increase nitrite production in CDNOS1KO mice (CDNOS1KO vehicle compared with dynasore P > 0.05); however, it was blunted in the CDNOS1KO IMCD. Two-factor ANOVA with Sidak's multiple (6) comparisons were used. *P < 0.05 compared with flox vehicle. †P < 0.05 compared with flox dynasore. C: flox control mice fed a HS diet for 7 days had significantly less inner medullary DNM2 than flox mice on NS diet or CDNOS1KO mice on HS diet (n = 4 mice per group). Two-factor ANOVA with Sidak's multiple (6) comparisons were used. *P < 0.05 compared with flox NS. ‡P < 005 compared with flox HS.
Similar articles
- Dynamin activates NO production in rat renal inner medullary collecting ducts via protein-protein interaction with NOS1.
Hyndman KA, Musall JB, Xue J, Pollock JS. Hyndman KA, et al. Am J Physiol Renal Physiol. 2011 Jul;301(1):F118-24. doi: 10.1152/ajprenal.00534.2010. Epub 2011 Apr 13. Am J Physiol Renal Physiol. 2011. PMID: 21490139 Free PMC article. - Distinct regulation of inner medullary collecting duct nitric oxide production from mice and rats.
Hyndman KA, Xue J, MacDonell A, Speed JS, Jin C, Pollock JS. Hyndman KA, et al. Clin Exp Pharmacol Physiol. 2013 Mar;40(3):233-9. doi: 10.1111/1440-1681.12057. Clin Exp Pharmacol Physiol. 2013. PMID: 23331097 Free PMC article. - Nitric oxide and the A and B of endothelin of sodium homeostasis.
Hyndman KA, Pollock JS. Hyndman KA, et al. Curr Opin Nephrol Hypertens. 2013 Jan;22(1):26-31. doi: 10.1097/MNH.0b013e32835b4edc. Curr Opin Nephrol Hypertens. 2013. PMID: 23160444 Free PMC article. Review. - Extracellular signal-regulated kinases 1/2 signaling pathways are not involved in endothelin regulation of mouse inner medullary collecting duct nitric oxide production.
Hyndman KA, MacDonell AH, Pollock JS. Hyndman KA, et al. Life Sci. 2012 Oct 15;91(13-14):578-82. doi: 10.1016/j.lfs.2012.01.014. Life Sci. 2012. PMID: 23293787 Free PMC article. - A review of Dynamin 2 involvement in cancers highlights a promising therapeutic target.
Trochet D, Bitoun M. Trochet D, et al. J Exp Clin Cancer Res. 2021 Jul 22;40(1):238. doi: 10.1186/s13046-021-02045-y. J Exp Clin Cancer Res. 2021. PMID: 34294140 Free PMC article. Review.
Cited by
- Collecting Duct Nitric Oxide Synthase 1ß Activation Maintains Sodium Homeostasis During High Sodium Intake Through Suppression of Aldosterone and Renal Angiotensin II Pathways.
Hyndman KA, Mironova EV, Giani JF, Dugas C, Collins J, McDonough AA, Stockand JD, Pollock JS. Hyndman KA, et al. J Am Heart Assoc. 2017 Oct 24;6(10):e006896. doi: 10.1161/JAHA.117.006896. J Am Heart Assoc. 2017. PMID: 29066445 Free PMC article. - Role of collecting duct principal cell NOS1β in sodium and potassium homeostasis.
Hyndman KA, Isaeva E, Palygin O, Mendoza LD, Rodan AR, Staruschenko A, Pollock JS. Hyndman KA, et al. Physiol Rep. 2021 Oct;9(20):e15080. doi: 10.14814/phy2.15080. Physiol Rep. 2021. PMID: 34665521 Free PMC article. - High salt induces autocrine actions of ET-1 on inner medullary collecting duct NO production via upregulated ETB receptor expression.
Hyndman KA, Dugas C, Arguello AM, Goodchild TT, Buckley KM, Burch M, Yanagisawa M, Pollock JS. Hyndman KA, et al. Am J Physiol Regul Integr Comp Physiol. 2016 Aug 1;311(2):R263-71. doi: 10.1152/ajpregu.00016.2015. Epub 2016 Jun 8. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27280426 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - The contribution of collecting duct NOS1 to the concentrating mechanisms in male and female mice.
Mendoza LD, Hyndman KA. Mendoza LD, et al. Am J Physiol Renal Physiol. 2019 Sep 1;317(3):F547-F559. doi: 10.1152/ajprenal.00180.2019. Epub 2019 Jun 26. Am J Physiol Renal Physiol. 2019. PMID: 31241990 Free PMC article.
References
- Armas-Padilla MC, Armas-Hernandez MJ, Sosa-Canache B, Cammarata R, Pacheco B, Guerrero J, Carvajal AR, Hernandez-Hernandez R, Israili ZH, Valasco M. Nitric oxide and malondialdehyde in human hypertension. Am J Ther 14: 172–176, 2007. - PubMed
- Baumann M, Schmaderer C, Kuznetsova T, Bartholome R, Smits JF, Richart T, Struijker-Boudier H, Staessen JA. Urinary nitric oxide metabolites and individual blood pressure progression to overt hypertension. Eur J Cardiovasc Prev Rehabil 18: 656–663, 2011. - PubMed
- Brenman JE, Xia H, Chao DS, Black SM, Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci 19: 224–231, 1997. - PubMed
- Cao S, Yao J, Shah V. The proline-rich domain of dynamin-2 is responsible for dynamin-dependent in vitro potentiation of endothelial nitric-oxide synthase activity via selective effects on reductase domain function. J Biol Chem 278: 5894–5901, 2003. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases