The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema - PubMed (original) (raw)
The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema
Stanley S Schwartz et al. Diabetes Care. 2016 Feb.
Abstract
The current classification system presents challenges to the diagnosis and treatment of patients with diabetes mellitus (DM), in part due to its conflicting and confounding definitions of type 1 DM, type 2 DM, and latent autoimmune diabetes of adults (LADA). The current schema also lacks a foundation that readily incorporates advances in our understanding of the disease and its treatment. For appropriate and coherent therapy, we propose an alternate classification system. The β-cell-centric classification of DM is a new approach that obviates the inherent and unintended confusions of the current system. The β-cell-centric model presupposes that all DM originates from a final common denominator-the abnormal pancreatic β-cell. It recognizes that interactions between genetically predisposed β-cells with a number of factors, including insulin resistance (IR), susceptibility to environmental influences, and immune dysregulation/inflammation, lead to the range of hyperglycemic phenotypes within the spectrum of DM. Individually or in concert, and often self-perpetuating, these factors contribute to β-cell stress, dysfunction, or loss through at least 11 distinct pathways. Available, yet underutilized, treatments provide rational choices for personalized therapies that target the individual mediating pathways of hyperglycemia at work in any given patient, without the risk of drug-related hypoglycemia or weight gain or imposing further burden on the β-cells. This article issues an urgent call for the review of the current DM classification system toward the consensus on a new, more useful system.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures
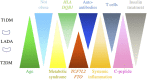
Figure 1
Qualitative illustration of the spectrum of factors associated with different forms of DM, including the variable age at onset, lack of obesity, metabolic syndrome, genetic associations, different forms of immune changes, C-peptide secretion, and the need for insulin therapy. T1DM, type 1 DM; T2DM, type 2 diabetes. Adapted with permission from Leslie et al. (1).
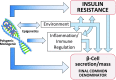
Figure 2
Genetic determinants influence IR (whether centrally or peripherally induced), loss of β-cell function and mass, environmental triggers (such as viruses, endocrine disruptors, food advanced glycosylation end products, gut biome), and immune modulation and inflammation. Singly or, more commonly, in various combinations, these factors converge on the genetically susceptible β-cell, impinge on β-cell function and biology, and orchestrate the shift from normoglycemia to hyperglycemia. As this process takes place regardless of subtype of DM, the dysfunctional β-cell is the final common denominator in all DM.
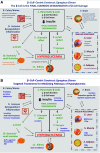
Figure 3
β-Cell–centric construct: the egregious eleven. Dysfunction of the β-cells is the final common denominator in DM. A: Eleven currently known mediating pathways of hyperglycemia are shown. Many of these contribute to β-cell dysfunction (liver, muscle, adipose tissue [shown in red to depict additional association with IR], brain, colon/biome, and immune dysregulation/inflammation [shown in blue]), and others result from β-cell dysfunction through downstream effects (reduced insulin, decreased incretin effect, α-cell defect, stomach/small intestine via reduced amylin, and kidney [shown in green]). B: Current targeted therapies for each of the current mediating pathways of hyperglycemia. GLP-1, glucagon-like peptide 1; QR, quick release.
Comment in
- Response to Comment on Schwartz et al. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016;39:179-186.
Schwartz SS, Epstein S, Corkey BE, Grant SF, Gavin JR 3rd, Aguilar RB. Schwartz SS, et al. Diabetes Care. 2016 Aug;39(8):e129-30. doi: 10.2337/dci16-0011. Diabetes Care. 2016. PMID: 27457644 No abstract available.
Similar articles
- [Autoimmune insulitis in patients with type 2 diabetes mellitus A randomized clinical trial in hospitalized patients].
Martinka E, Rončáková M, Mišániková M, Davani A. Martinka E, et al. Vnitr Lek. 2016 Fall;62(7-8):521-33. Vnitr Lek. 2016. PMID: 27627073 Clinical Trial. Czech. - Classification of diabetes.
Maraschin Jde F. Maraschin Jde F. Adv Exp Med Biol. 2012;771:12-9. doi: 10.1007/978-1-4614-5441-0_2. Adv Exp Med Biol. 2012. PMID: 23393666 Review. - Adult-onset autoimmune diabetes: current knowledge and implications for management.
Buzzetti R, Zampetti S, Maddaloni E. Buzzetti R, et al. Nat Rev Endocrinol. 2017 Nov;13(11):674-686. doi: 10.1038/nrendo.2017.99. Epub 2017 Sep 8. Nat Rev Endocrinol. 2017. PMID: 28885622 Review. - Modulation of the pancreatic islet-stress axis as a novel potential therapeutic target in diabetes mellitus.
Ludwig B, Barthel A, Reichel A, Block NL, Ludwig S, Schally AV, Bornstein SR. Ludwig B, et al. Vitam Horm. 2014;95:195-222. doi: 10.1016/B978-0-12-800174-5.00008-9. Vitam Horm. 2014. PMID: 24559919 Review. - Pramlintide acetate injection for the treatment of type 1 and type 2 diabetes mellitus.
Singh-Franco D, Robles G, Gazze D. Singh-Franco D, et al. Clin Ther. 2007 Apr;29(4):535-62. doi: 10.1016/j.clinthera.2007.04.005. Clin Ther. 2007. PMID: 17617279 Review.
Cited by
- Diabetic ketoacidosis during COVID-19 pandemic in a developing country.
Concepción Zavaleta MJ, Armas Flórez CD, Plasencia Dueñas EA, Coronado Arroyo JC. Concepción Zavaleta MJ, et al. Diabetes Res Clin Pract. 2020 Oct;168:108391. doi: 10.1016/j.diabres.2020.108391. Epub 2020 Aug 25. Diabetes Res Clin Pract. 2020. PMID: 32858095 Free PMC article. No abstract available. - Melatonin protects INS-1 pancreatic β-cells from apoptosis and senescence induced by glucotoxicity and glucolipotoxicity.
Lee YH, Jung HS, Kwon MJ, Jang JE, Kim TN, Lee SH, Kim MK, Park JH. Lee YH, et al. Islets. 2020 Jul 3;12(4):87-98. doi: 10.1080/19382014.2020.1783162. Epub 2020 Jul 16. Islets. 2020. PMID: 32673151 Free PMC article. - Personalized Type 2 Diabetes Management: An Update on Recent Advances and Recommendations.
Williams DM, Jones H, Stephens JW. Williams DM, et al. Diabetes Metab Syndr Obes. 2022 Feb 4;15:281-295. doi: 10.2147/DMSO.S331654. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 35153495 Free PMC article. Review. - Prevalence and associated factors of knee osteoarthritis in a rural Chinese adult population: an epidemiological survey.
Liu Y, Zhang H, Liang N, Fan W, Li J, Huang Z, Yin Z, Wu Z, Hu J. Liu Y, et al. BMC Public Health. 2016 Jan 30;16:94. doi: 10.1186/s12889-016-2782-x. BMC Public Health. 2016. PMID: 26830813 Free PMC article.
References
- Leslie RD, Palmer J, Schloot NC, Lernmark A. Diabetes at the crossroads: relevance of disease classification to pathophysiology and treatment. Diabetologia. 24 October 2015. [Epub ahead of print] - PubMed
- Grant SFA, Hakonarson H, Schwartz S. Can the genetics of type 1 and type 2 diabetes shed light on the genetics of latent autoimmune diabetes in adults? Endocr Rev 2010;31:183–193 - PubMed
- Rolandsson O, Palmer JP. Latent autoimmune diabetes in adults (LADA) is dead: long live autoimmune diabetes! Diabetologia 2010;53:1250–1253 - PubMed
- Basile KJ, Guy VC, Schwartz S, Grant SFA. Overlap of genetic susceptibility to type 1 diabetes, type 2 diabetes, and latent autoimmune diabetes in adults. Curr Diab Rep 2014;14:550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK035914/DK/NIDDK NIH HHS/United States
- DK99618/DK/NIDDK NIH HHS/United States
- R01 DK074778/DK/NIDDK NIH HHS/United States
- DK35914/DK/NIDDK NIH HHS/United States
- R01 DK099618/DK/NIDDK NIH HHS/United States
- DK56690/DK/NIDDK NIH HHS/United States
- R01 DK085212/DK/NIDDK NIH HHS/United States
- R56 DK035914/DK/NIDDK NIH HHS/United States
- R01 DK056690/DK/NIDDK NIH HHS/United States
- DK74778/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous