Stabilization of α-Synuclein Fibril Clusters Prevents Fragmentation and Reduces Seeding Activity and Toxicity - PubMed (original) (raw)
Stabilization of α-Synuclein Fibril Clusters Prevents Fragmentation and Reduces Seeding Activity and Toxicity
Huy T Lam et al. Biochemistry. 2016.
Abstract
Protein misfolding results in the accumulation of aggregated β-sheet-rich structures in Parkinson's disease (PD) and Alzheimer's disease. The toxic oligomer hypothesis stipulates that prefibrillar assemblies, such as soluble oligomers or protofibrils, are responsible for the poor prognosis of these diseases. Previous studies demonstrated that a small molecule related to the natural compound orcein, O4, directly binds to amyloid-β fibrils and stabilizes them, accelerating the formation of end-stage mature fibrils. Here we demonstrate a similar phenomenon during O4 treatment of α-synuclein (αsyn) aggregates, the protein responsible for PD pathology. While the drug did not change the kinetics of aggregate formation as measured by the amyloidophilic dye thioflavin T, O4 depleted αsyn oligomers and promoted the formation of sodium dodecyl sulfate and proteinase K resistant aggregates consisting of large fibril clusters. These fibril clusters exhibited reduced toxicity to human neuronal model cells and reduced seeding activity in vitro. The effectiveness of O4 decreased when it was added at later points in the αsyn aggregation pathway, which suggests that the incorporation of O4 into fibril assemblies stabilizes them against chemical, enzymatic, and mechanic degradation. These findings suggest that small molecules, which stabilize amyloid fibrils, can prevent fibril fragmentation and seeding and consequently prevent prion-like replication of misfolded αsyn. Inhibiting prion replication by fibril stabilization could thus be a therapeutic strategy for PD.
Figures
Figure 1
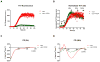
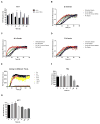
O4 does not alter the formation of β-sheet aggregates. (A) ThT fluorescence of αsyn (30μM) aggregation in the presence and absence of 1:1 O4 to αsyn under intermittent shaking conditions. The results represented mean ThT fluorescence intensities (n=3). (B) Normalized ThT fluorescence signals of αsyn samples in (A). Time points for further analysis: 6 h, 20 h, 48 h, 80 h, and 168 h were denoted. (C, D) CD spectra of αsyn incubated with O4 for 0 h and 48 h, respectively.
Figure 2
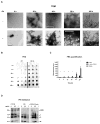
O4 induces SDS and protease resistant αsyn aggregates. (A) Transmission electron microscopy (TEM) of αsyn time points denoted in Fig. 1 in the presence or absence of O4; scale bar 500 nm. (B and C) Effect of O4 on SDS resistant aggregate formation quantified by FRA. The fluorescent signal was generated by immunostaining using anti-αsyn antibodies on a cellulose acetate membrane. (D) Effect of O4 on αsyn resistance to proteinase K digestion on 1 week time point samples. An additional sample was formed by incubating the αsyn control sample with equimolar O4 for 30 minutes to observe short-term effects of O4 treatment. HMW= High molecular weight, T=trimer, D= dimer, and M=monomer.
Figure 3
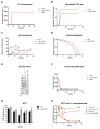
O4 Incubation rescues αsyn toxicity in vitro. (A) Time course of ThT fluorescence of αsyn under constant shaking condition. (B) ThT fluorescence data from (A) normalized to end point fluorescence. –(C) Aggregation time course monitored by light scattering. (D) Fraction of soluble αsyn analyzed densitometrically from SDS-PAGE after centrifugation at 200,000 × g. (E and F) Effects of O4 on the formation of αsyn oligomers as assessed by anti-oligomer antibody, A11, dot blot analysis. (G) Assessment of αsyn cytotoxicity using the MTT metabolic assay. SH-EP cells were incubated with αsyn time point samples (2.5μM) used in the A11 dot blot analysis for three days. *P<0.05, **P<0.01, ***P<0.001 (Student’s T-Test, n = 5. (H) Normalized MTT and A11 fluorescent signal data to compare time course dynamics.
Figure 4
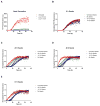
O4 Reduces seeding activity. (A) αsyn aggregation (30μM) monitored by Thioflavin T (ThT) fluorescence in the presence of 1:1 O4 to αsyn and 5:1 O4 to αsyn. Samples were taken at 6 h, 20 h, 48 h, and 72 h and sonicated to create seeds. (B - E); αsyn aggregation (30μM) in the presence of untreated seeds and O4 treated seeds created from the 6h, 20 h, 48 h, and 72 h samples, respectively (10% m/m). The results represented mean ThT fluorescence intensities (n=3).
Figure 5
Time dependent effect of O4 on forming SDS resistant aggregates and cellular toxicity. (A) Assessment of time point αsyn cytotoxicity using the MTT metabolic assay. SH-EP cells were incubated with αsyn time point samples (1.25μM) in the presence or absence of O4 for three days. The time points selected correspond to the same time points in Figure 1. *P<0.05, **P<0.01, ***P<0.001 (Student’s T-Test, n = 5). (B, C, and D) αsyn aggregation (30μM) in the presence of untreated seeds and O4 treated seeds created from the 20 h, 48 h, and 72 h samples, respectively (10% m/m). (E) ThT fluorescence of αsyn (30μM) aggregation with O4 added at different time points. The aggregation period lasted for 1 week. The results represented mean ThT fluorescence (n=3). (F) Effect of adding O4 at different time points during the aggregation period on SDS resistant aggregates quantified by FRA. The * on the 168 h time point indicates O4 treatment for 30 minutes. (G) Assessment of αsyn cytotoxicity using the MTT metabolic assay using the same samples (0.3 μM) used in the FRA (F). *P<0.05, **P<0.01, ***P<0.001 (Student’s T-Test, n = 3).
Figure 6
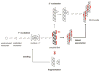
Model of autocatalytic amyloid formation through fibril fragmentation and secondary nucleation. O4 promotes the formation of fibril bundles and inhibits fragmentation and seeding.
Similar articles
- Heme Stabilization of α-Synuclein Oligomers during Amyloid Fibril Formation.
Hayden EY, Kaur P, Williams TL, Matsui H, Yeh SR, Rousseau DL. Hayden EY, et al. Biochemistry. 2015 Aug 4;54(30):4599-610. doi: 10.1021/acs.biochem.5b00280. Epub 2015 Jul 24. Biochemistry. 2015. PMID: 26161848 Free PMC article. - Structure of amyloid oligomers and their mechanisms of toxicities: Targeting amyloid oligomers using novel therapeutic approaches.
Salahuddin P, Fatima MT, Abdelhameed AS, Nusrat S, Khan RH. Salahuddin P, et al. Eur J Med Chem. 2016 May 23;114:41-58. doi: 10.1016/j.ejmech.2016.02.065. Epub 2016 Mar 2. Eur J Med Chem. 2016. PMID: 26974374 Review. - 14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic α-Synuclein.
Wang B, Underwood R, Kamath A, Britain C, McFerrin MB, McLean PJ, Volpicelli-Daley LA, Whitaker RH, Placzek WJ, Becker K, Ma J, Yacoubian TA. Wang B, et al. J Neurosci. 2018 Sep 19;38(38):8211-8232. doi: 10.1523/JNEUROSCI.1134-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093536 Free PMC article. - Effects of oligomer toxicity, fibril toxicity and fibril spreading in synucleinopathies.
Cascella R, Bigi A, Cremades N, Cecchi C. Cascella R, et al. Cell Mol Life Sci. 2022 Mar 4;79(3):174. doi: 10.1007/s00018-022-04166-9. Cell Mol Life Sci. 2022. PMID: 35244787 Free PMC article. Review. - N-Terminal Acetylation Affects α-Synuclein Fibril Polymorphism.
Watson MD, Lee JC. Watson MD, et al. Biochemistry. 2019 Sep 3;58(35):3630-3633. doi: 10.1021/acs.biochem.9b00629. Epub 2019 Aug 21. Biochemistry. 2019. PMID: 31424918 Free PMC article.
Cited by
- Neuromolecular imaging, a nanobiotechnology for Parkinson's disease: advancing pharmacotherapy for personalized medicine.
Broderick PA, Wenning L, Li YS. Broderick PA, et al. J Neural Transm (Vienna). 2017 Jan;124(1):57-78. doi: 10.1007/s00702-016-1633-3. Epub 2016 Oct 28. J Neural Transm (Vienna). 2017. PMID: 27796511 - Pyrogallol, Corilagin and Chebulagic acid target the "fuzzy coat" of alpha-synuclein to inhibit the fibrillization of the protein.
Bopardikar M, Koti Ainavarapu SR, Hosur RV. Bopardikar M, et al. RSC Adv. 2022 Dec 14;12(55):35770-35777. doi: 10.1039/d2ra04358k. eCollection 2022 Dec 12. RSC Adv. 2022. PMID: 36545068 Free PMC article. - Pathological TDP-43 changes in Betz cells differ from those in bulbar and spinal α-motoneurons in sporadic amyotrophic lateral sclerosis.
Braak H, Ludolph AC, Neumann M, Ravits J, Del Tredici K. Braak H, et al. Acta Neuropathol. 2017 Jan;133(1):79-90. doi: 10.1007/s00401-016-1633-2. Epub 2016 Oct 18. Acta Neuropathol. 2017. PMID: 27757524 Free PMC article. - Inhibition of protein misfolding and aggregation by natural phenolic compounds.
Dhouafli Z, Cuanalo-Contreras K, Hayouni EA, Mays CE, Soto C, Moreno-Gonzalez I. Dhouafli Z, et al. Cell Mol Life Sci. 2018 Oct;75(19):3521-3538. doi: 10.1007/s00018-018-2872-2. Epub 2018 Jul 20. Cell Mol Life Sci. 2018. PMID: 30030591 Free PMC article. Review. - The Contribution of _α_-Synuclein Spreading to Parkinson's Disease Synaptopathy.
Longhena F, Faustini G, Missale C, Pizzi M, Spano P, Bellucci A. Longhena F, et al. Neural Plast. 2017;2017:5012129. doi: 10.1155/2017/5012129. Epub 2017 Jan 3. Neural Plast. 2017. PMID: 28133550 Free PMC article. Review.
References
- Rao JN, Dua V, Ulmer TS. Characterization of alpha-synuclein interactions with selected aggregation-inhibiting small molecules. Biochemistry. 2008;47:4651–4656. - PubMed
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous