ChREBP Regulates Itself and Metabolic Genes Implicated in Lipid Accumulation in β-Cell Line - PubMed (original) (raw)
ChREBP Regulates Itself and Metabolic Genes Implicated in Lipid Accumulation in β-Cell Line
Chanachai Sae-Lee et al. PLoS One. 2016.
Abstract
Carbohydrate response element binding protein (ChREBP) is an important transcription factor that regulates a variety of glucose-responsive genes in hepatocytes. To date, only two natural isoforms, Chrebpα and Chrebpβ, have been identified. Although ChREBP is known to be expressed in pancreatic β cells, most of the glucose-responsive genes have never been verified as ChREBP targets in this organ. We aimed to explore the impact of ChREBP expression on regulating genes linked to accumulation of lipid droplets, a typical feature of β-cell glucotoxicity. We assessed gene expression in 832/13 cells overexpressing constitutively active ChREBP (caChREBP), truncated ChREBP with nearly identical amino acid sequence to Chrebpβ, or dominant negative ChREBP (dnChREBP). Among multiple ChREBP-controlled genes, ChREBP was sufficient and necessary for regulation of Eno1, Pklr, Mdh1, Me1, Pdha1, Acly, Acaca, Fasn, Elovl6, Gpd1, Cpt1a, Rgs16, Mid1ip1,Txnip, and Chrebpβ. Expression of Chrebpα and Srebp1c were not changed by caChREBP or dnChREBP. We identified functional ChREBP binding sequences that were located on the promoters of Chrebpβ and Rgs16. We also showed that Rgs16 overexpression lead to increased considerable amounts of lipids in 832/13 cells. This phenotype was accompanied by reduction of Cpt1a expression and slight induction of Fasn and Pklr gene in these cells. In summary, we conclude that Chrebpβ modulates its own expression, not that of Chrebpα; it also regulates the expression of several metabolic genes in β-cells without affecting SREBP-1c dependent regulation. We also demonstrate that Rgs16 is one of the ChREBP-controlled genes that potentiate accumulation of lipid droplets in β-cells.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
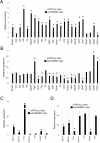
Fig 1. Exogenous ChREBP regulates Chrebpβ expression.
(A) Amino acid alignment among rat ChREBPβ, mouse ChREBPβ, caChREBP, and dnChREBP. GRACE, glucose response activation conserved element; bHLH, basic Helix-Loop-Helix-Leucine. (B) Schematic diagram of tetracycline-inducible vectors for overexpression of caChREBP, dnChREBP, and eYFPnuc. LTR, long terminal repeat; TRE, tetracycline-responsive promoter element; UBC, human ubiquitin C promoter; rtTA3, reverse tetracycline-transactivator 3; IRES, internal ribosomal entry site; Puro R, puromycin resistance gene; WPRE, Woodchuck hepatitis posttranscriptional regulatory element; SIN LTR, self-inactivating long terminal repeat. (C) caChREBP induces Chrebpβ expression. We incubated caChREBP cells for 48h in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. We isolated the RNA and performed RT-qPCR using ChREBP isoform-specific primers. The histograms are the means of relative RNA levels normalized to Eef1g and Rpl13a and expressed as fold activation over the activity seen in eYFPnuc cells incubated in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. *, p< 0.05 compared with eYFPnuc cells. (D) dnChREBP reduces the Chrebpβ expression. We preincubated dnChREBP cells for 24h in RPMI with 5.5 mmol/l D-glucose in the presence of doxycycline 1 μg/mL to minimize expression of endogenous nuclear ChREBP and let the induced dnChREBP occupy ChoREs and switched to in RPMI with 25 mmol/l D-glucose in the presence of doxycycline 1 μg/mL for 48h to induce strong expression and activity of endogenous nuclear ChREBP. We isolated the RNA and performed RT-qPCR using ChREBP isoform-specific primers. The histograms are the means of relative RNA levels normalized to Rns18 and Hprt1 and expressed as fold activation over the activity seen in eYFPnuc cells incubated under the same condition. *, p< 0.05 compared with eYFPnuc cells. (E) Alignment of ChoRE sequence presents in Chrebpβ promoter among rat (at the position -109 to -93), mouse, dog, horse, rhesus, and human. Sequence assemblies and coordinates are as follows: Rat: Mar.2012 Chr12(-): 26638089–26638073, Mouse: Jul.2007 Chr5(+): 135565651–135565667, Rhesus: Jan.2006 Chr3(-):51178915–51178899, Horse: Jan.2007 ChrUn(-):175282430–175282414, Dog: May 2005 Chr6(+): 9619423–9619439, Human: Mar.2006 Chr7(-):72700300–72700284. Color coding: light grey, identical residues; dark grey, unconserved residues. (F) Functional analysis of putative ChoRE sequences at the position -109 to -92 on Chrebpβ. We co-transfected luciferase reporter driven by two copies of rat Chrebpβ ChoRE upstream of minimal TATA promoter with caChREBP in 832/13 cells in RPMI with 5.5 mmol/l D-glucose. Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with two copies of ChoRE with minimal TATA promoter and empty vector. *, p< 0.05.
Fig 2. ChREBP regulates the expression of metabolic genes in 832/13 β-cells.
(A-B) Expression of genes encoding enzymes involved in glycolysis, lipogenesis, triglyceride biosynthesis and lipid oxidation in caChREBP cells (A) and dnChREBP cells (B). *p< 0.05 compared with eYFPnuc cells. (C-D) Expression of metabolic genes, including Rgs16 and Mid1ip1, Srebp1c and its target Irs2, and genes involved in cell proliferation, Hbegf and Myc, in caChREBP cells (C) and dnChREBP cells (D). *p< 0.05 compared with eYFPnuc cells.
Fig 3. Effect of caChREBP on Gpd1 or Rgs16 ChoRE-containing promoters.
(A) Alignment of ChoRE sequence presented on Gpd1 promoters among rat (at the position -1943 to -1927), mouse, cow, dog, rhesus, chimpanzee, and human. Sequence assemblies and coordinates are as follows: Rat: Mar.2012 ChrX(+): 115873245–11587361, Mouse: Dec.2011 Chr15(+): 99716109–99716125, Cow: Oct.2011 Chr5(-): 32732081–32732065, Dog: Sep.2011 Chr27(-): 4623314–4623298, Rhesus: Oct.2010 Chr11(+): 47359512–47359528, Chimpanzee: Feb.2011 Chr12(-): 39238594–39238578, and Human: Feb.2009 Chr12(+): 50496575–50496591. Color coding: light grey, identical residues; dark grey, unconserved residues. (B) caChREBP cannot activate Gpd1 ChoRE-containing promoter. We co-transfected luciferase reporter driven by two copies of rat Gpd1 ChoRE (position -1943 to -1927 relative to TSS) upstream of minimal TATA promoter with caChREBP in 832/13 cells in RPMI with 5.5 mmol/l D-glucose. Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with two copies of ChoRE with minimal TATA promoter and empty vector. *, p< 0.05. (C) Sequence of rat Rgs16 proximal promoter (-104 to +196, relative to TSS). Bold text, Start codon. Color coding: light grey, Exon 1. (D) Alignment of ChoRE sequence presented on Rgs16 promoter among rat (at the position +37 to +53), mouse, and human. Sequence assemblies and coordinates are as follows: Rat: Mar.2012 Chr13(+): 76145198–76145214, Mouse: Dec.2011 Chr1(+): 153740341–153740357, and Human: Dec.2013 Chr1(-): 182604401–182604385. Color coding: light grey, identical residues; dark grey, unconserved residues. (E) The effect of high glucose on the activity of natural Rgs16 promoter and mutated Rgs16 promoter. We transfected luciferase reporter driven by natural Rgs16 promoter (position -1519 to +159 relative to TSS) or ChoRE-deleted Rgs16 promoter in 832/13 cells cultured in RPMI with 5.5 mmol/l or 25 mmol/l D-glucose. Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with natural Rgs16 promoter and exposed to 5.5 mmol/l D-glucose. *, p< 0.05. (F-G) Glucose and caChREBP stimulates Rgs16 ChoRE-containing promoter. We transfected luciferase reporter driven by two copies of rat Rgs16 ChoRE (position +37 to +53 relative to TSS) upstream of minimal TATA promoter in 832/13 cells in RPMI with 5.5 or 25 mmol/l D-glucose (F) or co-transfected with caChREBP or empty vector in 832/13 cells in RPMI with 5.5 mmol/l D-glucose (G). Gaussia luciferase activity was measured at 48h and normalized to Cypridina luciferase, and expressed as fold activation over the activity seen in cells transfected with two copies of ChoRE with minimal TATA promoter and exposed to 5.5 mmol/l D-glucose (F) or co-transfected with empty vector (G). *, p< 0.05.
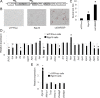
Fig 4. Rgs16 enhances accumulation of neutral lipid in 832/13 β-cells.
(A) Schematic diagram of tetracycline-inducible vectors for overexpression of Rgs16. LTR, long terminal repeat; TRE, tetracycline-responsive promoter element; UBC, human ubiquitin C promoter; rtTA3, reverse tetracycline-transactivator 3; IRES, internal ribosomal entry site; Puro R, puromycin resistance gene; WPRE, Woodchuck hepatitis posttranscriptional regulatory element; SIN LTR, self-inactivating long terminal repeat. (B-C) Overexpression of Rgs16 triggers lipid accumulation in 832/13 cells. We incubated eYFPnuc cells, Rgs16 cells, and caChREBP cells for 72h in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. We stained these cells for neutral lipid by Oil red O (B). Histograms (C) represent the amount of stained intracellular lipid compared with eYFPnuc cells. *, p< 0.05 compared with eYFPnuc cells. (D-E) Effects of Rgs16 overexpression on genes encoding metabolic enzymes (D) and related metabolic genes (E). We incubated Rgs16 cells for 72h in RPMI with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. We isolated the RNA and performed RT-qPCR using gene-specific primers. The histograms are the means of relative RNA levels normalized to Eef1g and Hprt1 and expressed as fold activation over the activity seen in eYFPnuc cells preincubated with 11 mmol/l D-glucose in the presence of doxycycline 1 μg/mL. *, p< 0.05 compared with eYFPnuc cells.
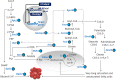
Fig 5. Model of ChREBP-induced lipid accumulation in β-cells.
Plus symbol, upregulation; Minus symbol, downregulation; Pgk1, phosphoglycerate kinase1; Eno1, enolase1; Pklr, L-type pyruvate kinase; Mdh1, malate dehydrogenase 1; Me1, malic enzyme1; Pdha1, pyruvate dehydrogenase (lipoamide) alpha 1; Cs, citrate synthase; Acly, ATP citrate lyase; Mid1ip1, MID1 interacting protein 1; Acaca, acetyl-CoA carboxylase alpha; Fasn, fatty acid synthase; Elovl6, ELOVL fatty acid elongase 6; Rgs16, regulator of G-protein signaling 16; Cpt1a, carnitine palmitoyltransferase 1; G-6-P, glucose-6-phosphate; G-3-P, glyceraldehydes-3-phosphate; 1,3-BPG, 1,3-bisphosphoglycerate; 3-PGA, 3-phosphoglycerate; 2-PGA, 2-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; DHAP, dihydroxyacetone phosphate; Glycerol-3-P, glycerol;-3-phosphate; OAA, oxaloacetate.
Similar articles
- Induction of the ChREBPβ Isoform Is Essential for Glucose-Stimulated β-Cell Proliferation.
Zhang P, Kumar A, Katz LS, Li L, Paulynice M, Herman MA, Scott DK. Zhang P, et al. Diabetes. 2015 Dec;64(12):4158-70. doi: 10.2337/db15-0239. Epub 2015 Sep 17. Diabetes. 2015. PMID: 26384380 Free PMC article. - Tissue Specific Effects of Dietary Carbohydrates and Obesity on ChREBPα and ChREBPβ Expression.
Stamatikos AD, da Silva RP, Lewis JT, Douglas DN, Kneteman NM, Jacobs RL, Paton CM. Stamatikos AD, et al. Lipids. 2016 Jan;51(1):95-104. doi: 10.1007/s11745-015-4090-0. Epub 2015 Nov 2. Lipids. 2016. PMID: 26526060 - Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity.
Poungvarin N, Lee JK, Yechoor VK, Li MV, Assavapokee T, Suksaranjit P, Thepsongwajja JJ, Saha PK, Oka K, Chan L. Poungvarin N, et al. Diabetologia. 2012 Jun;55(6):1783-96. doi: 10.1007/s00125-012-2506-4. Epub 2012 Mar 3. Diabetologia. 2012. PMID: 22382520 Free PMC article. - Adaptive and maladaptive roles for ChREBP in the liver and pancreatic islets.
Katz LS, Baumel-Alterzon S, Scott DK, Herman MA. Katz LS, et al. J Biol Chem. 2021 Jan-Jun;296:100623. doi: 10.1016/j.jbc.2021.100623. Epub 2021 Apr 2. J Biol Chem. 2021. PMID: 33812993 Free PMC article. Review. - ChREBP-Mediated Regulation of Lipid Metabolism: Involvement of the Gut Microbiota, Liver, and Adipose Tissue.
Iizuka K, Takao K, Yabe D. Iizuka K, et al. Front Endocrinol (Lausanne). 2020 Dec 3;11:587189. doi: 10.3389/fendo.2020.587189. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33343508 Free PMC article. Review.
Cited by
- Partitioning of MLX-Family Transcription Factors to Lipid Droplets Regulates Metabolic Gene Expression.
Mejhert N, Kuruvilla L, Gabriel KR, Elliott SD, Guie MA, Wang H, Lai ZW, Lane EA, Christiano R, Danial NN, Farese RV Jr, Walther TC. Mejhert N, et al. Mol Cell. 2020 Mar 19;77(6):1251-1264.e9. doi: 10.1016/j.molcel.2020.01.014. Epub 2020 Feb 4. Mol Cell. 2020. PMID: 32023484 Free PMC article. - Colocalization of MID1IP1 and c-Myc is Critically Involved in Liver Cancer Growth via Regulation of Ribosomal Protein L5 and L11 and CNOT2.
Jung JH, Lee HJ, Kim JH, Sim DY, Im E, Kim S, Chang S, Kim SH. Jung JH, et al. Cells. 2020 Apr 16;9(4):985. doi: 10.3390/cells9040985. Cells. 2020. PMID: 32316188 Free PMC article. - Pak1 mediates the stimulatory effect of insulin and curcumin on hepatic ChREBP expression.
Zeng K, Tian L, Sirek A, Shao W, Liu L, Chiang YT, Chernoff J, Ng DS, Weng J, Jin T. Zeng K, et al. J Mol Cell Biol. 2017 Oct 1;9(5):384-394. doi: 10.1093/jmcb/mjx031. J Mol Cell Biol. 2017. PMID: 28992163 Free PMC article. - Insulin-Responsive Transcription Factors.
Thiel G, Guethlein LA, Rössler OG. Thiel G, et al. Biomolecules. 2021 Dec 15;11(12):1886. doi: 10.3390/biom11121886. Biomolecules. 2021. PMID: 34944530 Free PMC article. Review. - The Protective Role of the Carbohydrate Response Element Binding Protein in the Liver: The Metabolite Perspective.
Agius L, Chachra SS, Ford BE. Agius L, et al. Front Endocrinol (Lausanne). 2020 Nov 17;11:594041. doi: 10.3389/fendo.2020.594041. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33281747 Free PMC article. Review.
References
- Decaux JF, Antoine B, Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989. July 15;264(20):11584–90. - PubMed
- Roche E, Farfari S, Witters LA, Assimacopoulos-Jeannet F, Thumelin S, Brun T, et al. Long-term exposure of beta-INS cells to high glucose concentrations increases anaplerosis, lipogenesis, and lipogenic gene expression. Diabetes. 1998. July;47(7):1086–94. - PubMed
- Prip-Buus C, Perdereau D, Foufelle F, Maury J, Ferre P, Girard J. Induction of fatty-acid-synthase gene expression by glucose in primary culture of rat hepatocytes. Dependency upon glucokinase activity. Eur J Biochem. 1995. May 15;230(1):309–15. - PubMed
- O'Callaghan BL, Koo SH, Wu Y, Freake HC, Towle HC. Glucose regulation of the acetyl-CoA carboxylase promoter PI in rat hepatocytes. J Biol Chem. 2001. May 11;276(19):16033–9. - PubMed
- Mourrieras F, Foufelle F, Foretz M, Morin J, Bouche S, Ferre P. Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations. Biochem J. 1997. September 1;326 (Pt 2):345–9. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL051586/HL/NHLBI NIH HHS/United States
- HL051586/HL/NHLBI NIH HHS/United States
- P30 DK079638/DK/NIDDK NIH HHS/United States
- P30DK079638/DK/NIDDK NIH HHS/United States
- DK105527/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous