Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children - PubMed (original) (raw)
Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children
Katri Korpela et al. Nat Commun. 2016.
Abstract
Early-life antibiotic use is associated with increased risk for metabolic and immunological diseases, and mouse studies indicate a causal role of the disrupted microbiome. However, little is known about the impacts of antibiotics on the developing microbiome of children. Here we use phylogenetics, metagenomics and individual antibiotic purchase records to show that macrolide use in 2-7 year-old Finnish children (N=142; sampled at two time points) is associated with a long-lasting shift in microbiota composition and metabolism. The shift includes depletion of Actinobacteria, increase in Bacteroidetes and Proteobacteria, decrease in bile-salt hydrolase and increase in macrolide resistance. Furthermore, macrolide use in early life is associated with increased risk of asthma and predisposes to antibiotic-associated weight gain. Overweight and asthmatic children have distinct microbiota compositions. Penicillins leave a weaker mark on the microbiota than macrolides. Our results support the idea that, without compromising clinical practice, the impact on the intestinal microbiota should be considered when prescribing antibiotics.
Figures
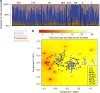
Figure 1. Microbiota composition in 257 fecal samples as arranged per group.
C denotes control group, no antibiotics for the past 2 years and in total <1 course per year of life on average. E denotes early-life exposure group, no antibiotics for the past 2 years and >1 course per year of life on average. M6 denotes macrolide course within 6 months; M12 denotes macrolide course within 6–12 months; M24 denotes macrolide course within 12–24 months. P6, P12 and P24 denote penicillin courses within 6, 6–12 and 12–24 months, respectively. (a) Phyla composition. (b) Genus-level microbiota composition according to PCoA analysis. The background colour indicates interpolated time since the last macrolide course.
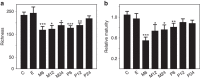
Figure 2. Microbiota richness and maturity relative to age in the different groups.
(a) Number of species-like phylotypes. (b) Score based on 24 age-associated genera (Supplementary Table 2). Asterisks indicate significance of the difference to the control group ‘C'. *P<0.05, **P<0.01 and ***P<0.001, estimated using linear models. Mean values are shown and the error bars show the s.e. values. The number of samples in both panels is 257 (Supplementary Table 1).
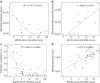
Figure 3. Macrolide resistance and bile-salt hydrolase abundance in relation to time since the last macrolide course.
The dashed lines show the model fit (linear or polynomial), _R_2 indicates the variation explained by the model and the P values (estimated using linear models) are indicated. (a) Macrolide resistance potential inferred from metagenomic analysis, _N_=14. (b) Bile-salt hydrolase abundance in metagenomes, _N_=14. (c) Macrolide resistance measured as proportion of anaerobic c.f.u.'s growing with erythromycin compared with c.f.u. without erythromycin, _N_=80. (d) Combined relative abundance of three bile-salt hydrolase genes (bsh) based on qPCR as a function of time since last macrolide course, _N_=37.
Comment in
- Antibiotics, gut bugs and the young.
Browne H. Browne H. Nat Rev Microbiol. 2016 Jun;14(6):336. doi: 10.1038/nrmicro.2016.73. Epub 2016 May 3. Nat Rev Microbiol. 2016. PMID: 27140687 - Antibiotic use in childhood alters the gut microbiota and predisposes to overweight.
Korpela K, de Vos WM. Korpela K, et al. Microb Cell. 2016 Jun 20;3(7):296-298. doi: 10.15698/mic2016.07.514. Microb Cell. 2016. PMID: 28357367 Free PMC article.
Similar articles
- Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding: Role of the Intestinal Microbiota.
Korpela K, Salonen A, Virta LJ, Kekkonen RA, de Vos WM. Korpela K, et al. JAMA Pediatr. 2016 Aug 1;170(8):750-7. doi: 10.1001/jamapediatrics.2016.0585. JAMA Pediatr. 2016. PMID: 27294842 Clinical Trial. - Antibiotics, gut bugs and the young.
Browne H. Browne H. Nat Rev Microbiol. 2016 Jun;14(6):336. doi: 10.1038/nrmicro.2016.73. Epub 2016 May 3. Nat Rev Microbiol. 2016. PMID: 27140687 - Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates.
Nogacka A, Salazar N, Suárez M, Milani C, Arboleya S, Solís G, Fernández N, Alaez L, Hernández-Barranco AM, de Los Reyes-Gavilán CG, Ventura M, Gueimonde M. Nogacka A, et al. Microbiome. 2017 Aug 8;5(1):93. doi: 10.1186/s40168-017-0313-3. Microbiome. 2017. PMID: 28789705 Free PMC article. - Antibiotics and the Intestinal Microbiome : Individual Responses, Resilience of the Ecosystem, and the Susceptibility to Infections.
Thiemann S, Smit N, Strowig T. Thiemann S, et al. Curr Top Microbiol Immunol. 2016;398:123-146. doi: 10.1007/82_2016_504. Curr Top Microbiol Immunol. 2016. PMID: 27738912 Review. - Macrolide and fluoroquinolone mediated cardiac arrhythmias: clinical considerations and comprehensive review.
Cornett E, Novitch MB, Kaye AD, Pann CA, Bangalore HS, Allred G, Bral M, Jhita PK, Kaye AM. Cornett E, et al. Postgrad Med. 2017 Sep;129(7):715-724. doi: 10.1080/00325481.2017.1362938. Epub 2017 Aug 10. Postgrad Med. 2017. PMID: 28770640 Review.
Cited by
- A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis.
Ozkul C, Ruiz VE, Battaglia T, Xu J, Roubaud-Baudron C, Cadwell K, Perez-Perez GI, Blaser MJ. Ozkul C, et al. Genome Med. 2020 Jul 25;12(1):65. doi: 10.1186/s13073-020-00764-z. Genome Med. 2020. PMID: 32711559 Free PMC article. - Deciphering the Role of the Gut Microbiota in Exposure to Emerging Contaminants and Diabetes: A Review.
Li X, Niu H, Huang Z, Zhang M, Xing M, Chen Z, Wu L, Xu P. Li X, et al. Metabolites. 2024 Feb 6;14(2):108. doi: 10.3390/metabo14020108. Metabolites. 2024. PMID: 38393000 Free PMC article. Review. - Population-level faecal metagenomic profiling as a tool to predict antimicrobial resistance in Enterobacterales isolates causing invasive infections: An exploratory study across Cambodia, Kenya, and the UK.
Auguet OT, Niehus R, Gweon HS, Berkley JA, Waichungo J, Njim T, Edgeworth JD, Batra R, Chau K, Swann J, Walker SA, Peto TEA, Crook DW, Lamble S, Turner P, Cooper BS, Stoesser N. Auguet OT, et al. EClinicalMedicine. 2021 May 30;36:100910. doi: 10.1016/j.eclinm.2021.100910. eCollection 2021 Jun. EClinicalMedicine. 2021. PMID: 34124634 Free PMC article. - Antibiotics at birth and later antibiotic courses: effects on gut microbiota.
Ainonen S, Tejesvi MV, Mahmud MR, Paalanne N, Pokka T, Li W, Nelson KE, Salo J, Renko M, Vänni P, Pirttilä AM, Tapiainen T. Ainonen S, et al. Pediatr Res. 2022 Jan;91(1):154-162. doi: 10.1038/s41390-021-01494-7. Epub 2021 Apr 6. Pediatr Res. 2022. PMID: 33824448 Free PMC article. - Causality of small and large intestinal microbiota in weight regulation and insulin resistance.
Scheithauer TP, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Scheithauer TP, et al. Mol Metab. 2016 Jun 10;5(9):759-70. doi: 10.1016/j.molmet.2016.06.002. eCollection 2016 Sep. Mol Metab. 2016. PMID: 27617199 Free PMC article.
References
- Chai G. et al.. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics 130, 23–31 (2012). - PubMed
- Virta L., Auvinen A., Helenius H., Huovinen P. & Kolho K. Association of repeated exposure to antibiotics with the development of pediatric Crohn's disease-a nationwide, register-based Finnish Case-Control Study. Am. J. Epidemiol. 175, 775–784 (2012). - PubMed
- Bailey L. C. et al.. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 168, 1063–1069 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical