Phase of Spontaneous Slow Oscillations during Sleep Influences Memory-Related Processing of Auditory Cues - PubMed (original) (raw)
Phase of Spontaneous Slow Oscillations during Sleep Influences Memory-Related Processing of Auditory Cues
Laura J Batterink et al. J Neurosci. 2016.
Abstract
Slow oscillations during slow-wave sleep (SWS) may facilitate memory consolidation by regulating interactions between hippocampal and cortical networks. Slow oscillations appear as high-amplitude, synchronized EEG activity, corresponding to upstates of neuronal depolarization and downstates of hyperpolarization. Memory reactivations occur spontaneously during SWS, and can also be induced by presenting learning-related cues associated with a prior learning episode during sleep. This technique, targeted memory reactivation (TMR), selectively enhances memory consolidation. Given that memory reactivation is thought to occur preferentially during the slow-oscillation upstate, we hypothesized that TMR stimulation effects would depend on the phase of the slow oscillation. Participants learned arbitrary spatial locations for objects that were each paired with a characteristic sound (eg, cat-meow). Then, during SWS periods of an afternoon nap, one-half of the sounds were presented at low intensity. When object location memory was subsequently tested, recall accuracy was significantly better for those objects cued during sleep. We report here for the first time that this memory benefit was predicted by slow-wave phase at the time of stimulation. For cued objects, location memories were categorized according to amount of forgetting from pre- to post-nap. Conditions of high versus low forgetting corresponded to stimulation timing at different slow-oscillation phases, suggesting that learning-related stimuli were more likely to be processed and trigger memory reactivation when they occurred at the optimal phase of a slow oscillation. These findings provide insight into mechanisms of memory reactivation during sleep, supporting the idea that reactivation is most likely during cortical upstates.
Significance statement: Slow-wave sleep (SWS) is characterized by synchronized neural activity alternating between active upstates and quiet downstates. The slow-oscillation upstates are thought to provide a window of opportunity for memory consolidation, particularly conducive to cortical plasticity. Recent evidence shows that sensory cues associated with previous learning can be delivered subtly during SWS to selectively enhance memory consolidation. Our results demonstrate that this behavioral benefit is predicted by slow-oscillation phase at stimulus presentation time. Cues associated with high versus low forgetting based on analysis of subsequent recall performance were delivered at opposite slow-oscillation phases. These results provide evidence of an optimal slow-oscillation phase for memory consolidation during sleep, supporting the idea that memory processing occurs preferentially during cortical upstates.
Keywords: memory consolidation; memory reactivation; phase; slow oscillation; slow-wave sleep.
Copyright © 2016 the authors 0270-6474/16/361401-09$15.00/0.
Figures
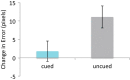
Figure 1.
Behavioral results showed significant cueing effect at the item level, with cued items showing less forgetting from pre- to post-nap compared with uncued items. Positive error scores indicate more forgetting from pre- to post-nap. Error bars indicate SEM (based on pooled means where the number of observations is equal to the total number of trials across all participants).
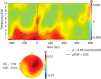
Figure 2.
Phase bifurcation index (ϕ) averaged across all channels, calculated over all pooled trials. A positive ϕ value indicates that cued items with high versus low pre- to post-nap forgetting show phase locking at opposite phase angles. Positive ϕ values were observed in the delta range (0.5–4 Hz) during the prestimulus interval (−400 to 0 ms) and continuing into the poststimulus interval. The dashed outermost contour line indicates that the ϕ at each time and frequency bin within the enclosed region is significant using an uncorrected one-tailed test (p < 0.05). To correct for multiple comparisons within our hypothesized time and frequency range (prestimulus interval from −400 to 0 ms; 0.5–4 Hz), the solid innermost contour line represents a region with significant ϕ that satisfies an FDR of 5%. The topographical plot on a schematic head as viewed from above shows the distribution of ϕ computed from 0.5 to 1 Hz and from −100 to 0 ms relative to stimulus onset (the time and frequency range where ϕ was maximal).
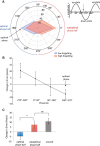
Figure 3.
Relationship between slow oscillation phase and associated memory performance for individual sound cues presented during sleep. A, Circular histogram of phases for each condition (more forgetting, less forgetting) averaged from 0.5 to 1 Hz and from −100 to 0 ms, across all scalp channels. Phases were pooled into six bins. Each corner represents the center of each phase angle bin (0°, 60°, 120°, 180°, 240°, and 300°). The distance of each corner from the origin represents the number of items within each phase bin that showed low (blue) and high (red) pre- to post-nap forgetting, divided by median split on a per-subject basis and pooled across all subjects. Optimal phase is defined as the phase range showing the highest proportion of items with low forgetting compared with items with high forgetting. Arrows indicate the approximate semicircle phase range associated with optimal (blue) and suboptimal (red) memory performance. The inset illustrates the correspondence between phase angle and slow oscillation upstates and downstates. The vertical arrow indicates the approximate phase range that may be optimal for auditory cues presentation. B, Change in spatial error from pre- to post-nap as a function of phase. Phases were pooled into four bins. This analysis suggests that the optimal phase for memory consolidation occurs between 180° and 270°. Error bars represent SEM. C, Bar graph showing change in spatial error between cued items presented during the optimal half of the phase range (90°–270°), cued items presented during the suboptimal half of the phase range (270°–90°), and uncued items. Items presented during the optimal phase half of the circle showed significantly improved performance from pre- to post-nap relative to items presented during the suboptimal phase half. There was no significant difference in performance between cued suboptimal phase items and uncued items. Error bars represent SEM.
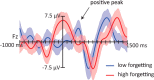
Figure 4.
Grand average ERPs from the Fz location time locked to sound cues presented during SWS, computed separately for objects that showed less forgetting (blue) versus more forgetting (red) pre- to post-nap. Trials were divided on the basis of median split by subject. A low-pass filter of 4 Hz was applied. On average, sound cues associated with more forgetting were presented at the peak of the positive half-wave, whereas the items associated with less forgetting were presented during the positive going slope of the slow oscillation, shortly before the next positive peak. Shaded regions around waveforms represent error bars (SEM).
Similar articles
- Targeted memory reactivation during sleep to strengthen memory for arbitrary pairings.
Vargas IM, Schechtman E, Paller KA. Vargas IM, et al. Neuropsychologia. 2019 Feb 18;124:144-150. doi: 10.1016/j.neuropsychologia.2018.12.017. Epub 2018 Dec 21. Neuropsychologia. 2019. PMID: 30582944 - Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation.
Cairney SA, Durrant SJ, Hulleman J, Lewis PA. Cairney SA, et al. Sleep. 2014 Apr 1;37(4):701-7, 707A. doi: 10.5665/sleep.3572. Sleep. 2014. PMID: 24688163 Free PMC article. - Targeted Memory Reactivation during Sleep Depends on Prior Learning.
Creery JD, Oudiette D, Antony JW, Paller KA. Creery JD, et al. Sleep. 2015 May 1;38(5):755-63. doi: 10.5665/sleep.4670. Sleep. 2015. PMID: 25515103 Free PMC article. Clinical Trial. - Slow oscillations orchestrating fast oscillations and memory consolidation.
Mölle M, Born J. Mölle M, et al. Prog Brain Res. 2011;193:93-110. doi: 10.1016/B978-0-444-53839-0.00007-7. Prog Brain Res. 2011. PMID: 21854958 Review. - How Targeted Memory Reactivation Promotes the Selective Strengthening of Memories in Sleep.
Lewis PA, Bendor D. Lewis PA, et al. Curr Biol. 2019 Sep 23;29(18):R906-R912. doi: 10.1016/j.cub.2019.08.019. Curr Biol. 2019. PMID: 31550479 Review.
Cited by
- Targeted memory reactivation during sleep influences social bias as a function of slow-oscillation phase and delta power.
Xia T, Antony JW, Paller KA, Hu X. Xia T, et al. Psychophysiology. 2023 May;60(5):e14224. doi: 10.1111/psyp.14224. Epub 2022 Dec 2. Psychophysiology. 2023. PMID: 36458473 Free PMC article. - Multielectrode Cortical Stimulation Selectively Induces Unidirectional Wave Propagation of Excitatory Neuronal Activity in Biophysical Neural Model.
Halgren AS, Siegel Z, Golden R, Bazhenov M. Halgren AS, et al. J Neurosci. 2023 Apr 5;43(14):2482-2496. doi: 10.1523/JNEUROSCI.1784-21.2023. Epub 2023 Feb 27. J Neurosci. 2023. PMID: 36849415 Free PMC article. - Sleep Promotes, and Sleep Loss Inhibits, Selective Changes in Firing Rate, Response Properties and Functional Connectivity of Primary Visual Cortex Neurons.
Clawson BC, Durkin J, Suresh AK, Pickup EJ, Broussard CG, Aton SJ. Clawson BC, et al. Front Syst Neurosci. 2018 Sep 7;12:40. doi: 10.3389/fnsys.2018.00040. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30245617 Free PMC article. - Closed-Loop Targeted Memory Reactivation during Sleep Improves Spatial Navigation.
Shimizu RE, Connolly PM, Cellini N, Armstrong DM, Hernandez LT, Estrada R, Aguilar M, Weisend MP, Mednick SC, Simons SB. Shimizu RE, et al. Front Hum Neurosci. 2018 Feb 6;12:28. doi: 10.3389/fnhum.2018.00028. eCollection 2018. Front Hum Neurosci. 2018. PMID: 29467633 Free PMC article. - Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans.
Geva-Sagiv M, Mankin EA, Eliashiv D, Epstein S, Cherry N, Kalender G, Tchemodanov N, Nir Y, Fried I. Geva-Sagiv M, et al. Nat Neurosci. 2023 Jun;26(6):1100-1110. doi: 10.1038/s41593-023-01324-5. Epub 2023 Jun 1. Nat Neurosci. 2023. PMID: 37264156 Free PMC article.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.
Publication types
MeSH terms
Grants and funding
- F32 HD078223/HD/NICHD NIH HHS/United States
- T32 NS047987/NS/NINDS NIH HHS/United States
- F32 HD 078223/HD/NICHD NIH HHS/United States
- T32 NS 047987/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous