The Human Microcirculation: Regulation of Flow and Beyond - PubMed (original) (raw)
Review
The Human Microcirculation: Regulation of Flow and Beyond
David D Gutterman et al. Circ Res. 2016.
Abstract
The microcirculation is responsible for orchestrating adjustments in vascular tone to match local tissue perfusion with oxygen demand. Beyond this metabolic dilation, the microvasculature plays a critical role in modulating vascular tone by endothelial release of an unusually diverse family of compounds including nitric oxide, other reactive oxygen species, and arachidonic acid metabolites. Animal models have provided excellent insight into mechanisms of vasoregulation in health and disease. However, there are unique aspects of the human microcirculation that serve as the focus of this review. The concept is put forth that vasculoparenchymal communication is multimodal, with vascular release of nitric oxide eliciting dilation and preserving normal parenchymal function by inhibiting inflammation and proliferation. Likewise, in disease or stress, endothelial release of reactive oxygen species mediates both dilation and parenchymal inflammation leading to cellular dysfunction, thrombosis, and fibrosis. Some pathways responsible for this stress-induced shift in mediator of vasodilation are proposed. This paradigm may help explain why microvascular dysfunction is such a powerful predictor of cardiovascular events and help identify new approaches to treatment and prevention.
Keywords: microcirculation; muscle, smooth, vascular; nitric oxide; oxidative stress; vasodilation.
© 2016 American Heart Association, Inc.
Figures
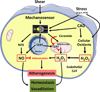
Figure 1
In a healthy heart (left), arteriolar endothelium produces NO, prostacyclin, and/or epoxyeicosatrienoic acids as well as low levels of hydrogen peroxide, which support a quiescent non-proliferative state. With onset of disease, flow through the microvasculature releases hydrogen peroxide, creating a pro-inflammatory environment throughout the organ, potentially leading to hypertrophy, fibrosis, and atherosclerosis.
Figure 2
Proposed mechanism for the stress-induced switch in the mediator of flow-induced dilation. In arterioles from healthy subjects, shear activates production of NO to stimulate dilation and vascular homeostasis (left side of diagram). Vascular stress or presence of coronary disease stimulates pathological basal levels of oxidants and initiates a switch in the mediator of flow-induced dilation from NO to hydrogen peroxide. This switch requires ceramide and a reduction in telomerase. Dilation is maintained but at the expense of vascular inflammation and its consequences.
Similar articles
- Critical Role for Telomerase in the Mechanism of Flow-Mediated Dilation in the Human Microcirculation.
Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Beyer AM, et al. Circ Res. 2016 Mar 4;118(5):856-66. doi: 10.1161/CIRCRESAHA.115.307918. Epub 2015 Dec 23. Circ Res. 2016. PMID: 26699654 Free PMC article. - Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity.
Ishizaka H, Kuo L. Ishizaka H, et al. Am J Physiol. 1997 Jul;273(1 Pt 2):H104-12. doi: 10.1152/ajpheart.1997.273.1.H104. Am J Physiol. 1997. PMID: 9249480 - Aged garlic extract improves homocysteine-induced endothelial dysfunction in macro- and microcirculation.
Weiss N, Ide N, Abahji T, Nill L, Keller C, Hoffmann U. Weiss N, et al. J Nutr. 2006 Mar;136(3 Suppl):750S-754S. doi: 10.1093/jn/136.3.750S. J Nutr. 2006. PMID: 16484556 - Microvascular Vasodilator Plasticity After Acute Exercise.
Robinson AT, Fancher IS, Mahmoud AM, Phillips SA. Robinson AT, et al. Exerc Sport Sci Rev. 2018 Jan;46(1):48-55. doi: 10.1249/JES.0000000000000130. Exerc Sport Sci Rev. 2018. PMID: 28816705 Free PMC article. Review. - Communication Is Key: Mechanisms of Intercellular Signaling in Vasodilation.
Freed JK, Gutterman DD. Freed JK, et al. J Cardiovasc Pharmacol. 2017 May;69(5):264-272. doi: 10.1097/FJC.0000000000000463. J Cardiovasc Pharmacol. 2017. PMID: 28482351 Free PMC article. Review.
Cited by
- Advances in Diagnosis and Pathophysiology of Microvascular Dysfunction.
Jan YK. Jan YK. Diagnostics (Basel). 2022 Mar 2;12(3):620. doi: 10.3390/diagnostics12030620. Diagnostics (Basel). 2022. PMID: 35328172 Free PMC article. - Association of Hyperhomocysteinemia with Increased Coronary Microcirculatory Resistance and Poor Short-Term Prognosis of Patients with Acute Myocardial Infarction after Elective Percutaneous Coronary Intervention.
Peng YP, Huang MY, Xue YJ, Pan JL, Lin C. Peng YP, et al. Biomed Res Int. 2020 Jan 2;2020:1710452. doi: 10.1155/2020/1710452. eCollection 2020. Biomed Res Int. 2020. PMID: 31998781 Free PMC article. Clinical Trial. - Microvascular Adaptations to Exercise: Protective Effect of PGC-1 Alpha.
Kadlec AO, Barnes C, Durand MJ, Gutterman DD. Kadlec AO, et al. Am J Hypertens. 2018 Jan 12;31(2):240-246. doi: 10.1093/ajh/hpx162. Am J Hypertens. 2018. PMID: 29140431 Free PMC article. - Efficacy of stimulating Mingmen (GV4) and Guanyuan (CV4) on kidney deficiency in rat model: laser irradiation traditional moxibustion.
Jing F, Wen P, Xiangyun W, Fengxing LI, Ling Z, Zouqin H, Xueyong S. Jing F, et al. J Tradit Chin Med. 2022 Dec;42(6):972-979. doi: 10.19852/j.cnki.jtcm.2022.06.008. J Tradit Chin Med. 2022. PMID: 36378056 Free PMC article. - Tobacco Use and Periodontal Disease-The Role of Microvascular Dysfunction.
Silva H. Silva H. Biology (Basel). 2021 May 17;10(5):441. doi: 10.3390/biology10050441. Biology (Basel). 2021. PMID: 34067557 Free PMC article. Review.
References
- Avakian A, Kalina RE, Sage EH, Rambhia AH, Elliott KE, Chuang EL, Clark JI, Hwang JN, Parsons-Wingerter P. Fractal analysis of region-based vascular change in the normal and non-proliferative diabetic retina. Curr Eye Res. 2002;24:274–280. - PubMed
- Nagaoka T, Yoshida A. Relationship between retinal fractal dimensions and retinal circulation in patients with type 2 diabetes mellitus. Curr Eye Res. 2013;38:1148–1152. - PubMed
- Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. - PubMed
- Brush JE, Jr, Cannon RO, 3rd, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988;319:1302–1307. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL135901/HL/NHLBI NIH HHS/United States
- R21 OD018306/OD/NIH HHS/United States
- R01 HL113612/HL/NHLBI NIH HHS/United States
- R01 HL080704/HL/NHLBI NIH HHS/United States
- R01 HL133029/HL/NHLBI NIH HHS/United States
- T32 GM089586/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources