No Fabry Disease in Patients Presenting with Isolated Small Fiber Neuropathy - PubMed (original) (raw)
No Fabry Disease in Patients Presenting with Isolated Small Fiber Neuropathy
Bianca T A de Greef et al. PLoS One. 2016.
Abstract
Objective: Screening for Fabry disease in patients with small fiber neuropathy has been suggested, especially since Fabry disease is potentially treatable. However, the diagnostic yield of testing for Fabry disease in isolated small fiber neuropathy patients has never been systematically investigated. Our aim is to determine the presence of Fabry disease in patients with small fiber neuropathy.
Methods: Patients referred to our institute, who met the criteria for isolated small fiber neuropathy were tested for Fabry disease by measurement of alpha-Galactosidase A activity in blood, lysosomal globotriaosylsphingosine in urine and analysis on possible GLA gene mutations.
Results: 725 patients diagnosed with small fiber neuropathy were screened for Fabry disease. No skin abnormalities were seen except for redness of the hands or feet in 30.9% of the patients. Alfa-Galactosidase A activity was tested in all 725 patients and showed diminished activity in eight patients. Lysosomal globotriaosylsphingosine was examined in 509 patients and was normal in all tested individuals. Screening of GLA for mutations was performed for 440 patients, including those with diminished α-Galactosidase A activity. Thirteen patients showed a GLA gene variant. One likely pathogenic variant was found in a female patient. The diagnosis Fabry disease could not be confirmed over time in this patient. Eventually none of the patients were diagnosed with Fabry disease.
Conclusions: In patients with isolated small fiber neuropathy, and no other signs compatible with Fabry disease, the diagnostic yield of testing for Fabry disease is extremely low. Testing for Fabry disease should be considered only in cases with additional characteristics, such as childhood onset, cardiovascular disease, renal failure, or typical skin lesions.
Conflict of interest statement
Competing Interests: The authors of this manuscript have the following competing interests: BTAG reports a grant from the Prinses Beatrix Spierfonds (W.OR12-01), outside the submitted work. JGJH reports no disclosure. EEW reports no disclosure. HJMS reports no disclosure. AW reports no disclosure. ISJM reports grants from GBS/CIDP foundation international for PeriNomS study and from European Union 7th Framework Programme (grant agreement no. 602273, 2013); Participation in steering committees of the Talecris ICE Study, CSL Behring, LFB, Novartis and Octapharma (a research foundation at the University of Maastricht received the honoraria on behalf of dr. Merkies), outside the submitted work. CGF reports grants from European Union 7th Framework Programme (grant agreement no. 602273, 2013), outside the submitted work, and the Prinses Beatrix Spierfonds (W.OR12-01), outside the submitted work. MMG reports a grant from European Union 7th Framework Programme (grant agreement no. 602273, 2013), outside the submitted work. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures
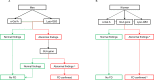
Fig 1. Diagnostic algorithm for confirming Fabry disease in SFN patients.
(A) Diagnostic algorithm for men. (B) Diagnostic algorithm for women. α-Gal A: α-galactosidase A, FD: Fabry disease, Lyso-GB3: lysosomal globotriaosylceramide. a Abnormal findings of the GLA gene include class 3 variants (uncertain to be pathogenic), class 4 variants (likely to be pathogenic), and class 5 variants (certain pathogenic). b The diagnosis FD is confirmed in women when the abnormal findings of the GLA gene complemented with abnormal findings in the biochemical assessment (α-Gal A and Lyso-GB3).
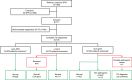
Fig 2. Small fiber neuropathy patients analyzed for Fabry disease in the Maastricht University Medical Center.
Illustration of the outcome of investigations to confirm the diagnosis of Fabry disease. α-Gal A: α-galactosidase A, Lyso-GB3: lysosomal globotriaosylceramide, FD: Fabry disease, SFN: small fiber neuropathy. a Missing data. b The measurement of lyso-GB3 excretion in urine was incorporated in our workflow for SFN patients from April 2012. c GLA gene sequencing was performed in all women, and in males in case of reduced α-Gal A enzyme activity. d These includes the class 2 variants (unlikely to be pathogenic) and the class 3 variants (uncertain to be pathogenic).
Similar articles
- Phenotypical characterization of α-galactosidase A gene mutations identified in a large Fabry disease screening program in stroke in the young.
De Brabander I, Yperzeele L, Ceuterick-De Groote C, Brouns R, Baker R, Belachew S, Delbecq J, De Keulenaer G, Dethy S, Eyskens F, Fumal A, Hemelsoet D, Hughes D, Jeangette S, Nuytten D, Redondo P, Sadzot B, Sindic C, Sheorajpanday R, Thijs V, Van Broeckhoven C, De Deyn PP. De Brabander I, et al. Clin Neurol Neurosurg. 2013 Jul;115(7):1088-93. doi: 10.1016/j.clineuro.2012.11.003. Epub 2012 Dec 4. Clin Neurol Neurosurg. 2013. PMID: 23219219 - Diagnosis of Fabry Disease Using Alpha-Galactosidase A Activity or LysoGb3 in Blood Fails to Identify Up to Two Thirds of Female Patients.
Duro G, Anania M, Zizzo C, Francofonte D, Giacalone I, D'Errico A, Marsana EM, Colomba P. Duro G, et al. Int J Mol Sci. 2024 May 9;25(10):5158. doi: 10.3390/ijms25105158. Int J Mol Sci. 2024. PMID: 38791200 Free PMC article. - Plasma Globotriaosylsphingosine and α-Galactosidase A Activity as a Combined Screening Biomarker for Fabry Disease in a Large Japanese Cohort.
Maruyama H, Taguchi A, Mikame M, Izawa A, Morito N, Izaki K, Seto T, Onishi A, Sugiyama H, Sakai N, Yamabe K, Yokoyama Y, Yamashita S, Satoh H, Toyoda S, Hosojima M, Ito Y, Tazawa R, Ishii S. Maruyama H, et al. Curr Issues Mol Biol. 2021 Jun 19;43(1):389-404. doi: 10.3390/cimb43010032. Curr Issues Mol Biol. 2021. PMID: 34205365 Free PMC article. - Fabry disease.
Toyooka K. Toyooka K. Handb Clin Neurol. 2013;115:629-42. doi: 10.1016/B978-0-444-52902-2.00037-0. Handb Clin Neurol. 2013. PMID: 23931807 Review. - Fabry Disease and Central Nervous System Involvement: From Big to Small, from Brain to Synapse.
Cortés-Saladelafont E, Fernández-Martín J, Ortolano S. Cortés-Saladelafont E, et al. Int J Mol Sci. 2023 Mar 9;24(6):5246. doi: 10.3390/ijms24065246. Int J Mol Sci. 2023. PMID: 36982318 Free PMC article. Review.
Cited by
- Scientific Advances in and Clinical Approaches to Small-Fiber Polyneuropathy: A Review.
Oaklander AL, Nolano M. Oaklander AL, et al. JAMA Neurol. 2019 Oct 1;76(10):1240-1251. doi: 10.1001/jamaneurol.2019.2917. JAMA Neurol. 2019. PMID: 31498378 Free PMC article. - Molecular Aspects of Regional Pain Syndrome.
Baronio M, Sadia H, Paolacci S, Prestamburgo D, Miotti D, Guardamagna VA, Natalini G, Sullivan SGB, Bertelli M. Baronio M, et al. Pain Res Manag. 2020 Apr 11;2020:7697214. doi: 10.1155/2020/7697214. eCollection 2020. Pain Res Manag. 2020. PMID: 32351641 Free PMC article. Review. - Fabry Disease: Recognition, Diagnosis, and Treatment of Neurological Features.
Ranieri M, Bedini G, Parati EA, Bersano A. Ranieri M, et al. Curr Treat Options Neurol. 2016 Jul;18(7):33. doi: 10.1007/s11940-016-0414-5. Curr Treat Options Neurol. 2016. PMID: 27225543 Review. - Genetic studies of human neuropathic pain conditions: a review.
Zorina-Lichtenwalter K, Parisien M, Diatchenko L. Zorina-Lichtenwalter K, et al. Pain. 2018 Mar;159(3):583-594. doi: 10.1097/j.pain.0000000000001099. Pain. 2018. PMID: 29240606 Free PMC article. Review. - Associated conditions in small fiber neuropathy - a large cohort study and review of the literature.
de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. de Greef BTA, et al. Eur J Neurol. 2018 Feb;25(2):348-355. doi: 10.1111/ene.13508. Epub 2017 Dec 18. Eur J Neurol. 2018. PMID: 29112785 Free PMC article. Review.
References
- Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of tests of sweating and autonomic cardiovascular reflexes. Muscle Nerve. 1992;15(6):661–5. . - PubMed
- Gorson KC, Ropper AH. Idiopathic distal small fiber neuropathy. Acta Neurol Scand. 1995;92(5):376–82. . - PubMed
- Gorson KC, Herrmann DN, Thiagarajan R, Brannagan TH, Chin RL, Kinsella LJ, et al. Non-length dependent small fibre neuropathy/ganglionopathy. J Neurol Neurosurg Psychiatry. 2008;79(2):163–9. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous