Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study - PubMed (original) (raw)
Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study
Magnus Gisslén et al. EBioMedicine. 2015.
Erratum in
- Corrigendum to: "Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: A cross-sectional study" [EBioMedicine 3 (216) 135-140].
Gisslén M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H. Gisslén M, et al. EBioMedicine. 2016 May;7:287-288. doi: 10.1016/j.ebiom.2016.04.021. Epub 2016 Apr 20. EBioMedicine. 2016. PMID: 27322482 Free PMC article. No abstract available.
Abstract
Background: Cerebrospinal fluid (CSF) neurofilament light chain protein (NFL) is a sensitive marker of neuronal injury in a variety of neurodegenerative conditions, including the CNS dysfunction injury that is common in untreated HIV infection. However, an important limitation is the requirement for lumbar puncture. For this reason, a sensitive and reliable blood biomarker of CNS injury would represent a welcome advance in both clinical and research settings.
Methods: To explore whether plasma concentrations of NFL might be used to detect CNS injury in HIV infection, an ultrasensitive Single molecule array (Simoa) immunoassay was developed. Using a cross-sectional design, we measured NFL in paired CSF and plasma samples from 121 HIV-infected subjects divided into groups according to stage of their systemic disease, presence of overt HIV-associated dementia (HAD), and after antiretroviral treatment (ART)-induced viral suppression. HIV-negative controls were also examined.
Findings: Plasma and CSF NFL concentrations were very highly correlated (r = 0.89, P < 0.0001). While NFL was more than 50-fold lower plasma than CSF it was within the quantifiable range of the new plasma assay in all subjects, including the HIV negatives and the HIV positives with normal CSF NFL concentrations. The pattern of NFL changes were almost identical in plasma and CSF, both exhibiting similar age-related increases in concentrations along with highest values in HAD and substantial elevations in ART-naïve neuroasymptomatic subjects with low blood CD4(+) T cells.
Interpretation: These results show that plasma NFL may prove a valuable tool to evaluate ongoing CNS injury in HIV infection that may be applied in the clinic and in research settings to assess the presence if active CNS injury. Because CSF NFL is also elevated in a variety of other CNS disorders, sensitive measures of plasma NFL may similarly prove useful in other settings.
Keywords: Antiretroviral Treatment; Biomarker; CNS; CSF; Central Nervous System; Cerebrospinal Fluid; HAD; HIV; HIV Associated Dementia; NFL; Neurofilament Light Chain; Plasma.
Figures
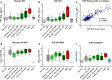
Fig. 1
Association between CSF and plasma NFL and concentrations of biomarkers in 8 subject groups. Panels a–b and d–f, plot concentrations of different markers for the 8 subject groups, HIV-negative controls (HIV-neg); primary HIV infection (PHI); untreated neuroasymptomatic subjects in different CD4 cell strata (Asympt); HIV-associated dementia (HAD); and subjects on suppressive antiretroviral treatment (ART). Boxes depict median and IQR, whiskers show 10–90 percentiles and ‘+’ designates the means. Dotted horizontal lines in panels d–e show the limit of detection for HIV RNA (40 copies/mL), and in panel f the upper limit of normal for CSF neopterin (5.8 nmol/L). Panel c plots the correlation between log CSF and log plasma NFL.
Fig. 2
Association between NFL and age. Panel a displays the regression lines for age to log plasma and log CSF NFL in HIV-negative controls. Age was closely correlated with plasma NFL (r = 0.79, p < 0.0001).
Comment in
- Plasma Biomarkers of HIV-associated Cognitive Disease.
Winston A, Underwood J. Winston A, et al. EBioMedicine. 2015 Nov 28;3:12-13. doi: 10.1016/j.ebiom.2015.11.052. eCollection 2016 Jan. EBioMedicine. 2015. PMID: 26870812 Free PMC article. No abstract available.
Similar articles
- CSF neurofilament protein (NFL) -- a marker of active HIV-related neurodegeneration.
Abdulle S, Mellgren A, Brew BJ, Cinque P, Hagberg L, Price RW, Rosengren L, Gisslén M. Abdulle S, et al. J Neurol. 2007 Aug;254(8):1026-32. doi: 10.1007/s00415-006-0481-8. Epub 2007 Apr 10. J Neurol. 2007. PMID: 17420923 - Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy.
Guha D, Mukerji SS, Chettimada S, Misra V, Lorenz DR, Morgello S, Gabuzda D. Guha D, et al. AIDS. 2019 Mar 15;33(4):615-625. doi: 10.1097/QAD.0000000000002121. AIDS. 2019. PMID: 30557159 Free PMC article. - Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection.
Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, Yiannoutsos CT, Spudich SS, Price RW. Peterson J, et al. PLoS One. 2014 Dec 26;9(12):e116081. doi: 10.1371/journal.pone.0116081. eCollection 2014. PLoS One. 2014. PMID: 25541953 Free PMC article. - Neurofilament light chain protein as a marker of neuronal injury: review of its use in HIV-1 infection and reference values for HIV-negative controls.
Yilmaz A, Blennow K, Hagberg L, Nilsson S, Price RW, Schouten J, Spudich S, Underwood J, Zetterberg H, Gisslén M. Yilmaz A, et al. Expert Rev Mol Diagn. 2017 Aug;17(8):761-770. doi: 10.1080/14737159.2017.1341313. Epub 2017 Jun 14. Expert Rev Mol Diagn. 2017. PMID: 28598205 Review. - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
- Non-classical monocyte levels correlate negatively with HIV-associated cerebral small vessel disease and cognitive performance.
Singh MV, Uddin MN, Covacevich Vidalle M, Sutton KR, Boodoo ZD, Peterson AN, Tyrell A, Tivarus ME, Wang HZ, Sahin B, Zhong J, Weber MT, Wang L, Qiu X, Maggirwar SB, Schifitto G. Singh MV, et al. Front Cell Infect Microbiol. 2024 Oct 23;14:1405431. doi: 10.3389/fcimb.2024.1405431. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39507948 Free PMC article. - Elevated plasma and CSF neurofilament light chain concentrations are stabilized in response to mutant huntingtin lowering in the brains of Huntington's disease mice.
Caron NS, Byrne LM, Lemarié FL, Bone JN, Aly AE, Ko S, Anderson C, Casal LL, Hill AM, Hawellek DJ, McColgan P, Wild EJ, Leavitt BR, Hayden MR. Caron NS, et al. Transl Neurodegener. 2024 Oct 8;13(1):50. doi: 10.1186/s40035-024-00443-8. Transl Neurodegener. 2024. PMID: 39380076 Free PMC article. - Impact of APOE ε4 and ε2 on plasma neurofilament light chain and cognition in autosomal dominant Alzheimer's disease.
Langella S, Bonta K, Chen Y, Su Y, Vasquez D, Aguillon D, Acosta-Baena N, Baena AY, Garcia-Ospina G, Giraldo-Chica M, Tirado V, Muñoz C, Ríos-Romenets S, Guzman-Martínez C, Pruzin JJ, Ghisays V, Arboleda-Velasquez JF, Kosik KS, Tariot PN, Reiman EM, Lopera F, Quiroz YT. Langella S, et al. Alzheimers Res Ther. 2024 Oct 1;16(1):208. doi: 10.1186/s13195-024-01572-y. Alzheimers Res Ther. 2024. PMID: 39354618 Free PMC article. - Neurofilament Light Chain as Biomarker in Encephalitis.
Wellmann S, Geis T, Kuhle J, Lehnerer V. Wellmann S, et al. J Clin Med. 2024 Sep 12;13(18):5416. doi: 10.3390/jcm13185416. J Clin Med. 2024. PMID: 39336905 Free PMC article. - Changes in cerebrospinal fluid proteins across the spectrum of untreated and treated chronic HIV-1 infection.
Hu Z, Cinque P, Dravid A, Hagberg L, Yilmaz A, Zetterberg H, Fuchs D, Gostner J, Blennow K, Spudich SS, Kincer L, Zhou S, Joseph SB, Swanstrom R, Price RW, Gisslén M. Hu Z, et al. PLoS Pathog. 2024 Sep 24;20(9):e1012470. doi: 10.1371/journal.ppat.1012470. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39316609 Free PMC article.
References
- Anon. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41(6):778–785. - PubMed
- Canestri A., Lescure F.X., Jaureguiberry S. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010;50(5):773–778. - PubMed
- Constantinescu R., Rosengren L., Johnels B., Zetterberg H., Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical Parkinsonian disorders. Parkinsonism Relat. Disord. 2010;16(2):142–145. - PubMed
- Gisslen M., Fuchs D., Svennerholm B., Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 1999;21(4):271–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21MH096619/MH/NIMH NIH HHS/United States
- P01MH094177/MH/NIMH NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- R01 MH062701/MH/NIMH NIH HHS/United States
- R21 MH096619/MH/NIMH NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- R01MH62701/MH/NIMH NIH HHS/United States
- P01 MH094177/MH/NIMH NIH HHS/United States
- R01 NS094067/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials