MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4 - PubMed (original) (raw)
. 2016 Feb 23;14(7):1590-1601.
doi: 10.1016/j.celrep.2016.01.057. Epub 2016 Feb 11.
Nicholas A Graham 2, Wen Gu 1, Carolina Espindola Camacho 1, Vei Mah 3, Erin L Maresh 3, Mohammed Alavi 3, Lora Bagryanova 3, Pascal A L Krotee 1, Brian K Gardner 4, Iman Saramipoor Behbahan 5, Steve Horvath 6, David Chia 7, Ingo K Mellinghoff 8, Sara A Hurvitz 9, Steven M Dubinett 10, Susan E Critchlow 11, Siavash K Kurdistani 12, Lee Goodglick 7, Daniel Braas 13, Thomas G Graeber 14, Heather R Christofk 15
Affiliations
- PMID: 26876179
- PMCID: PMC4816454
- DOI: 10.1016/j.celrep.2016.01.057
MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4
Candice Sun Hong et al. Cell Rep. 2016.
Abstract
Monocarboxylate transporter 1 (MCT1) inhibition is thought to block tumor growth through disruption of lactate transport and glycolysis. Here, we show MCT1 inhibition impairs proliferation of glycolytic breast cancer cells co-expressing MCT1 and MCT4 via disruption of pyruvate rather than lactate export. MCT1 expression is elevated in glycolytic breast tumors, and high MCT1 expression predicts poor prognosis in breast and lung cancer patients. Acute MCT1 inhibition reduces pyruvate export but does not consistently alter lactate transport or glycolytic flux in breast cancer cells that co-express MCT1 and MCT4. Despite the lack of glycolysis impairment, MCT1 loss-of-function decreases breast cancer cell proliferation and blocks growth of mammary fat pad xenograft tumors. Our data suggest MCT1 expression is elevated in glycolytic cancers to promote pyruvate export that when inhibited, enhances oxidative metabolism and reduces proliferation. This study presents an alternative molecular consequence of MCT1 inhibitors, further supporting their use as anti-cancer therapeutics.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
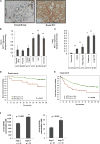
Figure 1. Unbiased gene expression analysis finds that MCT1 correlates with glycolytic phenotype in breast cancer
a, Breast tumors with high and low FDG uptake have distinct gene expression signatures. Transcript levels from 11 human breast cancers were ranked by the average correlation with tumor FDG maximum standardized uptake value (SUVmax) and cell line glycolytic phenotype (nmol lactate produced/nmol oxygen consumed) and arranged from left to right in order of increasing FDG uptake. Red and green denote high and low average correlation coefficients, respectively. MCT1 is the top-ranked of 13,374 genes. The inset table lists metabolic pathways enriched in highly glycolytic tumors and cell lines (PPP, pentose phosphate pathway; Glyc/Gluc, glycolysis/gluconeogenesis). b, Highly glycolytic tumors and cell lines demonstrate coordinate upregulation of glycolysis genes and MCT1. Genes within the glycolysis pathway are colored red or green to denote high and low correlation coefficients with glycolytic phenotype, respectively. c, Levels of MCT1, but not other MCT family members, are highly correlated with glycolytic phenotype in breast tumors and cell lines. The Pearson correlation coefficient with FDG uptake in human breast tumors (left) and glycolytic phenotype in human breast cancer cell lines (right) is depicted for microarray probes recognizing MCT1-4 family members. d, Scatter plots of MCT1 and MCT4 expression demonstrate that MCT1 but not MCT4 is highly correlated with glycolytic phenotypes. Transcript levels are plotted versus FDG uptake for human breast tumors (left) and glycolytic phenotype for human breast cancer cell lines (right). P-values are the two-tailed significance of the Pearson correlation coefficient. See also Figure S1.
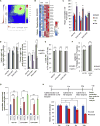
Figure 2. Elevated MCT1 levels are indicative of tumor malignancy and poor breast and lung cancer patient survival
a, Immunohistochemistry of human normal breast and breast invasive ductal carcinoma (IDC) with an antibody towards MCT1 indicates higher MCT1 expression in the malignant tissue. Images are shown at X 100 magnification. b, The mean integrated MCT1 expression as determined by immunohistochemistry on a breast tissue microarray is compared across breast histologies and histopathologies. MCT1 expression is significantly increased in ductal carcinoma in situ (DCIS, P = 0.003), invasive ductal carcinoma (IDC, P < 0.001), and lymph node metastatic lesions (LN mets, P = 0.004) compared to adjacent non-malignant glandular epithelium (“normal”) or epithelium from voluntary breast reductions (BR). There is no significant difference between normal breast glandular epithelium and ductal hyperplasia (Ductal Hyper.) lesions. **c**, The mean integrated MCT1 expression as determined by immunohistochemistry on a lung tissue microarrary is compared across lung histologies and histopathologies. MCT1 expression is significantly increased in adenocarcinoma (Adeno, P < 0.001), adeno-squamous (Adeno-Squam, P < 0.001), squamous cell (P < 0.001) and large cell (P < 0.001) compared to adjacent non-malignant bronchial epithelium. Expression of MCT1 is significantly elevated in squamous and large cell tumors compared to adenocarcinomas and adeno-squamous tumors (P < 0.001 for both). For **b, c**, error bars denote standard error of the mean, and n = number of tissue array spots analyzed. **d**, Higher MCT1 expression levels predicts poorer survival in women with invasive ductal carcinoma of the breast. Kaplan-Meier survival plot shows patients with lower MCT1 expression (< 2.0 mean integrated intensity) depicted as a green line, and higher MCT1 expression (≥ 2.0 mean integrated intensity) depicted as a red line. **e**, Higher MCT1 expression levels predict poorer survival in individuals with NSCLC. Kaplan-Meier survival plot shows patients with lower MCT1 expression (≤ 1.0 mean integrated intensity) depicted as a green line and higher MCT1 expression (> 1.0 mean integrated intensity) depicted as a red line. f, Serum lactate (left) and pyruvate (right) concentrations from Stage I versus Stage IV lung cancer patients. For d, e, f, n = number of individuals in each category.
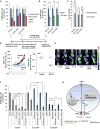
Figure 3. MCT1 is critical for pyruvate export in breast cancer cell lines
a, Gene expression profiles from SUM149PT cells expressing scrambled shRNA (control) and shRNA towards MCT1 (knockdown) were used to generate a ranked list of transcripts that are differentially expressed upon MCT1 knockdown. This ranked list was compared to a ranked list of transcripts that correlate with high FDG uptake in breast tumors from patients using the rank-rank hypergeometric overlap (RRHO) algorithm. The resulting overlap from the ranked lists, represented as a hypergeometric heat map, indicates that MCT1 knockdown renders glycolytic SUM149PT breast cancer cells less similar to tumors with high FDG uptake. The direction-signed log10-transformed hypergeometric p-values are indicated in the accompanying color scale. b, Heatmap indicating fold changes in expression levels of genes in the oxidative phosphorylation gene set from SUM149PT cells treated with DMSO or AZD3965 (MCT1i) for 24 hr. c, Cellular oxygen consumption rates 24 hrs post treatment with DMSO versus 250 nM AZD3965 (MCT1i). d, Lactate and pyruvate export rates 4 hours post treatment of the indicated cell lines with DMSO or 250 nM AZD3965 (MCT1i). e, Percentage of the 13C-labeled M1 lactate isotopomer from cells 24 hr post labeling with 1-13C-lactate and treatment with DMSO or 250 nM AZD3965 (MCT1i) in the presence or absence of 4 mM glutamine as determined by LC-MS/MS. f, Percentage of the 13C-labeled M2 lactate isotopomer from cells 24 hr post labeling with 1,2-13C-glucose and treatment with DMSO or 250 nM AZD3965 (MCT1i) as determined by LC-MS/MS. g, Intracellular pyruvate levels from the indicated cell lines treated with DMSO, 250 nM MCT1i, or 5 mM methyl-pyruvate for 30min. h, Relative abundance of media lactate and pyruvate post treatment of SUM149PT cells with 250 nM AZD3965 (MCT1i) for the indicated times as determined by GC-MS. Error bars in c denote standard error of mean (n=5). Error bars in d–g denote standard deviation (n=3). * denotes p < 0.05; ** denotes p < 0.01. See also Figure S2 and S3
Figure 4. MCT1 loss-of-function reduces breast cancer cell proliferation and tumor growth
a, Proliferation rates of the indicated breast cancer cell lines stably expressing shRNA that knocks down MCT1 expression (MCT1 shRNA) versus control scrambled shRNA (scramble shRNA), and vehicle (DMSO) treated cells versus 250 nM AZD3965 (MCT1i). b, Proliferation rates of the indicated cell lines treated for 4 days with DMSO or 5mM methyl-pyruvate. c, Percentage of MCT1 knockdown cells (MCT1 shRNA) versus control cells (scramble shRNA) in the G0/G1 phase of the cell cycle. d and e, Relative tumor volumes (d) and FDG-PET/CT images (e) from NSG mice with mammary fat pad xenograft tumors derived from SUM149PT cells treated by oral gavage twice daily with either 0.5% hydroxypropyl methyl cellulose/0.1%tween (vehicle) or 0.1ml/10g AZD3965 (MCT1i) for seven days. In (e) T indicates tumor, and values shown represent mean injected dose per gram (%ID/g) calculated from tumor regions of interest. f, Proliferation rates of indicated breast cancer cell lines after five days of treatment with DMSO, MCT1i (IC50), metformin (IC50), metformin + MCT1i (IC50 for both), or phenformin (IC50), phenformin + MCT1i (IC50 for both). MCT1i was replenished every day. g, Schematic representation of the impact of MCT1 inhibition on breast cancer cell metabolism and proliferation. AZD3965 consistently reduces pyruvate export, increases oxygen consumption, and reduces proliferation rates. Dual treatment of AZD3965 with metformin or phenformin further reduces proliferation rates, presumably by blocking the compensatory switch to oxidative metabolism caused by MCT1 inhibition to sustain proliferation. Error bars in a–c denote standard deviation n=3). Error bars in e denote standard error of mean. For a–c and f, * denotes p < 0.05, and ** denotes p < 0.01. See Figure S4.
Similar articles
- CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors.
Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouysségur J. Le Floch R, et al. Proc Natl Acad Sci U S A. 2011 Oct 4;108(40):16663-8. doi: 10.1073/pnas.1106123108. Epub 2011 Sep 19. Proc Natl Acad Sci U S A. 2011. PMID: 21930917 Free PMC article. - Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer.
Polański R, Hodgkinson CL, Fusi A, Nonaka D, Priest L, Kelly P, Trapani F, Bishop PW, White A, Critchlow SE, Smith PD, Blackhall F, Dive C, Morrow CJ. Polański R, et al. Clin Cancer Res. 2014 Feb 15;20(4):926-937. doi: 10.1158/1078-0432.CCR-13-2270. Epub 2013 Nov 25. Clin Cancer Res. 2014. PMID: 24277449 Free PMC article. - Disruption of BASIGIN decreases lactic acid export and sensitizes non-small cell lung cancer to biguanides independently of the LKB1 status.
Granja S, Marchiq I, Le Floch R, Moura CS, Baltazar F, Pouysségur J. Granja S, et al. Oncotarget. 2015 Mar 30;6(9):6708-21. doi: 10.18632/oncotarget.2862. Oncotarget. 2015. PMID: 25894929 Free PMC article. - Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights.
Puri S, Juvale K. Puri S, et al. Eur J Med Chem. 2020 Aug 1;199:112393. doi: 10.1016/j.ejmech.2020.112393. Epub 2020 May 1. Eur J Med Chem. 2020. PMID: 32388280 Review. - The expression of lactate transporters (MCT1 and MCT4) in heart and muscle.
Bonen A. Bonen A. Eur J Appl Physiol. 2001 Nov;86(1):6-11. doi: 10.1007/s004210100516. Eur J Appl Physiol. 2001. PMID: 11820324 Review.
Cited by
- LncRNA-SLC16A1-AS1 induces metabolic reprogramming during Bladder Cancer progression as target and co-activator of E2F1.
Logotheti S, Marquardt S, Gupta SK, Richter C, Edelhäuser BAH, Engelmann D, Brenmoehl J, Söhnchen C, Murr N, Alpers M, Singh KP, Wolkenhauer O, Heckl D, Spitschak A, Pützer BM. Logotheti S, et al. Theranostics. 2020 Jul 29;10(21):9620-9643. doi: 10.7150/thno.44176. eCollection 2020. Theranostics. 2020. PMID: 32863950 Free PMC article. - Interfering cellular lactate homeostasis overcomes Taxol resistance of breast cancer cells through the microRNA-124-mediated lactate transporter (MCT1) inhibition.
Hou L, Zhao Y, Song GQ, Ma YH, Jin XH, Jin SL, Fang YH, Chen YC. Hou L, et al. Cancer Cell Int. 2019 Jul 24;19:193. doi: 10.1186/s12935-019-0904-0. eCollection 2019. Cancer Cell Int. 2019. PMID: 31367191 Free PMC article. - Metabolic targeting of malignant tumors: a need for systemic approach.
Margetis AT. Margetis AT. J Cancer Res Clin Oncol. 2023 May;149(5):2115-2138. doi: 10.1007/s00432-022-04212-w. Epub 2022 Aug 4. J Cancer Res Clin Oncol. 2023. PMID: 35925428 Review. - Simultaneous targeting of glycolysis and oxidative phosphorylation as a therapeutic strategy to treat diffuse large B-cell lymphoma.
Noble RA, Thomas H, Zhao Y, Herendi L, Howarth R, Dragoni I, Keun HC, Vellano CP, Marszalek JR, Wedge SR. Noble RA, et al. Br J Cancer. 2022 Sep;127(5):937-947. doi: 10.1038/s41416-022-01848-w. Epub 2022 May 26. Br J Cancer. 2022. PMID: 35618788 Free PMC article. - Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies.
Zappasodi R, Merghoub T, Wolchok JD. Zappasodi R, et al. Cancer Cell. 2018 Apr 9;33(4):581-598. doi: 10.1016/j.ccell.2018.03.005. Cancer Cell. 2018. PMID: 29634946 Free PMC article. Review.
References
- Bonen A, Miskovic D, Tonouchi M, Lemieux K, Wilson MC, Marette A, Halestrap AP. Abundance and subcellular distribution of MCT1 and MCT4 in heart and fast-twitch skeletal muscles. American journal of physiology. Endocrinology and metabolism. 2000;278:E1067–1077. - PubMed
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van’t Veer LJ, Bartelink H, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102:3738–3743. - PMC - PubMed
- Chen H, Wang L, Beretov J, Hao J, Xiao W, Li Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin Exp Metastasis. 2010;27:557–569. - PubMed
- Choi JW, Kim Y, Lee JH, Kim YS. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology. 2014;84:245 e249–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA152751/CA/NCI NIH HHS/United States
- R25 CA098010/CA/NCI NIH HHS/United States
- UL1TR000124/TR/NCATS NIH HHS/United States
- CA86366/CA/NCI NIH HHS/United States
- U24 CA086366/CA/NCI NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- U54 CA151819/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 NS080944/NS/NINDS NIH HHS/United States
- P30 CA016042/CA/NCI NIH HHS/United States
- DP2 OD008454-01/OD/NIH HHS/United States
- R25T CA098010/CA/NCI NIH HHS/United States
- DP2 OD008454/OD/NIH HHS/United States
- P01 CA168585/CA/NCI NIH HHS/United States
- U01 CA152751/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical