The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults - PubMed (original) (raw)
Review
The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults
Małgorzata Nita et al. Oxid Med Cell Longev. 2016.
Abstract
The reactive oxygen species (ROS) form under normal physiological conditions and may have both beneficial and harmful role. We search the literature and current knowledge in the aspect of ROS participation in the pathogenesis of anterior and posterior eye segment diseases in adults. ROS take part in the pathogenesis of keratoconus, Fuchs endothelial corneal dystrophy, and granular corneal dystrophy type 2, stimulating apoptosis of corneal cells. ROS play a role in the pathogenesis of glaucoma stimulating apoptotic and inflammatory pathways on the level of the trabecular meshwork and promoting retinal ganglion cells apoptosis and glial dysfunction in the posterior eye segment. ROS play a role in the pathogenesis of Leber's hereditary optic neuropathy and traumatic optic neuropathy. ROS induce apoptosis of human lens epithelial cells. ROS promote apoptosis of vascular and neuronal cells and stimulate inflammation and pathological angiogenesis in the course of diabetic retinopathy. ROS are associated with the pathophysiological parainflammation and autophagy process in the course of the age-related macular degeneration.
Figures
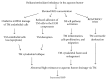
Figure 1
Schematic overview of the harmful influence of ROS and the oxidative stress on the trabecular meshwork structure and its function in glaucoma. ROS, reactive oxygen species; mtDNA, mitochondrial deoxyribonucleic acid; TM, trabecular meshwork; ECM, extracellular matrix; NF-_κ_B, nuclear factor-_κ_B; ROS/ONOO−, reactive oxygen species/peroxynitrite; IOP, intraocular pressure.
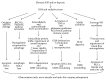
Figure 2
Schematic overview of the influence of ROS and the oxidative stress on the retina and the optic nerve head changes in the course of glaucomatous neurodegeneration. IOP, intraocular pressure; ROS, reactive oxygen species; mtDNA, mitochondrial deoxyribonucleic acid; RGCs, retinal ganglion cells; BECN1/PtdIns3K, Beclin 1/phosphatidylinositol 3-kinase; AGEs, advanced glycation end products; ONH, optic nerve head; TNF-α, tumor necrosis factor alpha; NO, nitric oxide; AGE/RAGE, advanced glycation end product/receptor for advanced glycation end product; MMPs, matrix metalloproteinases; ECM, extracellular matrix.
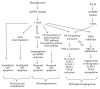
Figure 3
Schematic overview of the ROS influence on the development of microvascular complications, neurodegeneration, and pathological angiogenesis in the course of diabetic retinopathy. mtDNA, mitochondrial deoxyribonucleic acid; ROS, reactive oxygen species; Sirt6, the name of a nuclear chromatin-bound protein; RAS, renin-angiotensin system; BDNF, brain-derived neurotrophic factor; PKC, the protein kinase C, AGEs, advanced glycation end products; NF-_κ_B, nuclear factor-_κ_B; TNF-α, tumor necrosis factor alpha; IL-6, IL-8, interleukins 6 and 8; COX-2, cyclooxygenase 2; ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; VEGF, vascular endothelial growth factor; PHDs, prolyl hydroxylases; HIF-1, hypoxia-inducible factor-1; SCDF-1, stromal cell derived factor-1; RAAS, rennin-angiotensin-aldosterone system; NADPH-oxidase, nicotinamide adenine dinucleotide phosphate-oxidase.
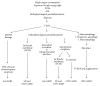
Figure 4
Schematic overview of the ROS influence on the development of early and advanced forms of age-related macular degeneration. PUFA, polyunsaturated fatty acid; A2E, a component of retinal pigmented epithelial cell (RPE) lipofuscin; ROS, reactive oxygen species; mtDNA, mitochondrial deoxyribonucleic acid; ECM, extracellular matrix; PECAM-1, platelet endothelial cell adhesion molecule; VEGF, vascular endothelial growth factor; SD, soft drusen; GA/AMD, geographic atrophy/age-related macular degeneration; CNV/AMD, choroidal neovascularization/age-related macular degeneration.
Similar articles
- Oxidative stress in the pathogenesis of keratoconus and Fuchs endothelial corneal dystrophy.
Wojcik KA, Kaminska A, Blasiak J, Szaflik J, Szaflik JP. Wojcik KA, et al. Int J Mol Sci. 2013 Sep 23;14(9):19294-308. doi: 10.3390/ijms140919294. Int J Mol Sci. 2013. PMID: 24065107 Free PMC article. Review. - DNA damage and repair in Fuchs endothelial corneal dystrophy.
Czarny P, Kasprzak E, Wielgorski M, Udziela M, Markiewicz B, Blasiak J, Szaflik J, Szaflik JP. Czarny P, et al. Mol Biol Rep. 2013 Apr;40(4):2977-83. doi: 10.1007/s11033-012-2369-2. Epub 2012 Dec 29. Mol Biol Rep. 2013. PMID: 23275192 Free PMC article. - Keratoconus and Fuchs' corneal endothelial dystrophy in a patient and her family.
Lipman RM, Rubenstein JB, Torczynski E. Lipman RM, et al. Arch Ophthalmol. 1990 Jul;108(7):993-4. doi: 10.1001/archopht.1990.01070090095047. Arch Ophthalmol. 1990. PMID: 2369360 - Keratan sulphate in the trabecular meshwork and cornea.
Davies Y, Fullwood NJ, Marcyniuk B, Bonshek R, Tullo A, Nieduszynski IA. Davies Y, et al. Curr Eye Res. 1997 Jul;16(7):677-86. doi: 10.1076/ceyr.16.7.677.5053. Curr Eye Res. 1997. PMID: 9222085 - Contributions of electron microscopy to the study of corneal pathology.
Polack FM. Polack FM. Surv Ophthalmol. 1976 May-Jun;20(6):375-414. doi: 10.1016/0039-6257(76)90066-7. Surv Ophthalmol. 1976. PMID: 779087 Review.
Cited by
- Oxidative stress in the eye and its role in the pathophysiology of ocular diseases.
Böhm EW, Buonfiglio F, Voigt AM, Bachmann P, Safi T, Pfeiffer N, Gericke A. Böhm EW, et al. Redox Biol. 2023 Dec;68:102967. doi: 10.1016/j.redox.2023.102967. Epub 2023 Nov 18. Redox Biol. 2023. PMID: 38006824 Free PMC article. Review. - Chemical Profiling and Dose-Dependent Assessment of Fear Reducing and Memory-Enhancing Effects of Solanum virginianum in Rats.
Javaid U, Javaid S, Ashraf W, Rasool MF, Noman OM, Alqahtani AS, Majeed A, Shakeel W, Albekairi TH, Alqahtani F, Imran I. Javaid U, et al. Dose Response. 2021 Mar 4;19(1):1559325821998486. doi: 10.1177/1559325821998486. eCollection 2021 Jan-Mar. Dose Response. 2021. PMID: 33746655 Free PMC article. - Glaucoma-TrEl: A web-based interactive database to build evidence-based hypotheses on the role of trace elements in glaucoma.
Choudhari JK, Eberhardt M, Chatterjee T, Hohberger B, Vera J. Choudhari JK, et al. BMC Res Notes. 2022 Nov 18;15(1):348. doi: 10.1186/s13104-022-06210-0. BMC Res Notes. 2022. PMID: 36401306 Free PMC article. - Efficacy of Alpinumisoflavone Isolated from Maclura tricuspidata Fruit in Tumor Necrosis Factor-α-Induced Damage of Human Dermal Fibroblasts.
Lee S, Hoang GD, Kim D, Song HS, Choi S, Lee D, Kang KS. Lee S, et al. Antioxidants (Basel). 2021 Mar 25;10(4):514. doi: 10.3390/antiox10040514. Antioxidants (Basel). 2021. PMID: 33806207 Free PMC article. - Promising Support Coming from Nature: Antioxidant and Anti-Inflammatory Potential of Castanea sativa Wood Distillate on Skin Cells.
Filippelli A, Ciccone V, Loppi S, Morbidelli L. Filippelli A, et al. Curr Issues Mol Biol. 2024 Aug 26;46(9):9386-9400. doi: 10.3390/cimb46090556. Curr Issues Mol Biol. 2024. PMID: 39329908 Free PMC article.
References
- Quinlan C. L., Goncalves R. L. S., Hey-Mogensen M., Yadava N., Bunik V. I., Brand M. D. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. The Journal of Biological Chemistry. 2014;289(12):8312–8325. doi: 10.1074/jbc.m113.545301. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical