The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation - PubMed (original) (raw)
Review
The ubiquitin signal and autophagy: an orchestrated dance leading to mitochondrial degradation
Koji Yamano et al. EMBO Rep. 2016 Mar.
Abstract
The quality of mitochondria, essential organelles that produce ATP and regulate numerous metabolic pathways, must be strictly monitored to maintain cell homeostasis. The loss of mitochondrial quality control systems is acknowledged as a determinant for many types of neurodegenerative diseases including Parkinson's disease (PD). The two gene products mutated in the autosomal recessive forms of familial early-onset PD, Parkin and PINK1, have been identified as essential proteins in the clearance of damaged mitochondria via an autophagic pathway termed mitophagy. Recently, significant progress has been made in understanding how the mitochondrial serine/threonine kinase PINK1 and the E3 ligase Parkin work together through a novel stepwise cascade to identify and eliminate damaged mitochondria, a process that relies on the orchestrated crosstalk between ubiquitin/phosphorylation signaling and autophagy. In this review, we highlight our current understanding of the detailed molecular mechanisms governing Parkin-/PINK1-mediated mitophagy and the evidences connecting Parkin/PINK1 function and mitochondrial clearance in neurons.
Keywords: PINK1; Parkin; Parkinson's disease; mitochondria; mitophagy.
© 2016 The Authors.
Figures
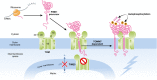
Figure 1. PINK1 accumulation in the outer membrane of the damaged mitochondria
The newly synthesized
PINK
1 precursor is targeted to the damaged mitochondria. Because of the loss of membrane potential, the
PINK
1 precursor is not allowed to enter the inner membrane via the
TIM
23 complex. Instead, the
PINK
1 precursor is inserted into the outer membrane through the
TOM
complex in a
TOMM
7‐dependent manner.
PINK
1 stabilized on the outer membrane then forms a large complex with the
TOM
complex and undergoes intermolecular autophosphorylation at residues S228 and S402. 5, 6, 7, 20, 22, 40, and 70 denote
TOMM
5,
TOMM
6,
TOMM
7,
TOMM
20,
TOMM
22,
TOMM
40, and
TOMM
70, respectively.
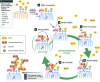
Figure 2. Positive feedback ubiquitination cycles induced by Parkin and ubiquitin chain formation on damaged mitochondria
Although most of the ubiquitin diffuses in the cytosol, a fraction should reside on the outer membrane of healthy mitochondria since the ubiquitin system also contributes to the turnover of mitochondrial proteins under normal conditions 178 (Step 1). Following dissipation of the membrane potential,
PINK
1 is stabilized on the damaged mitochondria (Step 2).
PINK
1 can then phosphorylate the ubiquitin that is conjugated to the mitochondrial proteins, or
PINK
1 may also phosphory‐late Ser65 of cytosolic Parkin (Step 3). Of note, phosphorylated ubiquitin stably stays on the mitochondria because hydrolysis of phosphorylated ubiquitin chain by
DUB
s is impaired 75. Through higher affinity with phosphorylated ubiquitin, cytosolic Parkin is recruited to and retained on the mitochondria (Step 4).
PINK
1 further phosphorylates Parkin on the mitochondria.
PINK
1 may also phosphorylate Ser65 of cytosolic ubiquitin (Step 5). The fully activated, phosphorylated Parkin can then elongate ubiquitin chains or generate a new ubiquitinated substrate from cytosolic‐free ubiquitin. In other words, cytosolic ubiquitin is recruited to the mitochon‐dria through a ubiquitination reaction by activated Parkin (Step 6). The ubiquitin on the mitochondrial substrate is phosphorylated by
PINK
1 (Step 7 is the next round of the Step 3). Positive feedback amplification cycles (Steps 3–7) result in both Parkin and ubiquitin recruitment to and poly‐ubiquitin chain formation on the damaged mitochondria (Step 8).
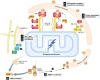
Figure 3. Ephemeral life of PINK1 in the healthy mitochondria
(A) The newly synthesized
PINK
1 precursor on the cytosolic ribosomes is targeted to the mitochondria. After crossing the outer membrane through the
TOM
complex, the N‐terminal mitochondrial targeting sequence is cleaved by
MPP
in the matrix. The following transmembrane segment is recognized by the
TIM
23 complex and received second cleavage by
PARL
between A103 and F104. Most of the cleaved
PINK
1 is released to the cytosol where the newly N‐terminal phenylalanine residue of the cleaved
PINK
1 is recognized by the N‐end rule
UBR
ligases (
UBR
1,
UBR
2, and
UBR
4 that preferentially recognize type‐2 N‐degrons) for proteasomal degradation. A matrix
ATP
ase associated with diverse cellular activities (m‐
AAA
) protease is composed of
AFG
3L2 and paraplegin and has the active site oriented toward the matrix. Clp
XP
is a matrix
ATP
‐dependent protease composed of hetero‐oligomeric
ATP
‐binding subunits and proteolytic subunits. m‐
AAA
and Clp
XP
also participate in
PINK
1 degradation. Another
ATP
‐dependent
LON
protease also contributes, particularly in Drosophila, to
PINK
1 degradation in the matrix. As phosphorylation of
NDUFA
10 in the respiratory chain complex I is reduced in _Pink1_‐
KO
mice, a small amount of
PINK
1 retained in the inner membrane might be involved in the maintenance of complex I through phosphorylation. (B) Amino acid sequence alignment of the transmembrane region of
PINK
1. Amino acid sequences of the predicted transmembrane segments (pink‐colored box) from the indicated species are shown 99. The transmembrane regions are well conserved from zebrafish (Danio rerio) and humans (Homo sapiens), while the transmembrane segment in fly (Drosophila melanogaster) is less conserved with fewer glycine/proline residues. The
PARL
cleavage site of human
PINK
1 between A103 and F104 is also shown.
Figure 4. Activation and recruitment of autophagy machineries during Parkin‐/PINK1‐mediated mitophagy
Following the generation of poly‐ubiquitin chains on damaged mitochondria, the indicated autophagy proteins including adaptors and regulators are recruited to the mitochondria in a multi‐independent process. (i) While autophagosomes form at
ER
–mitochondria contact sites under starvation‐induced autophagy 179, how the
ULK
1 and
PI
3K complexes and the omegasome marked by
DFCP
1 recognize mitochondrial damage remains unknown. (ii) Poly‐ubiquitin chains on the mitochondria are directly recognized by the autophagy adaptors, in particular
NDP
52 and optineurin (
OPTN
), which are phosphorylated by
TBK
1, and promote the recruitment of the
LC
3‐labeled isolation membrane. (iii) Atg9A vesicles are independently recruited to the mitochondria through an unknown mechanism. (iv) Mitochondria‐localized Rab‐
GAP
s,
TBC
1D15 and
TBC
1D17, via interaction of Fis1 regulate proper autophagosomal formation by modulating Rab7 activity. (v) Upon mitophagy stimulation, a regulator of autophagy‐lysosome biogenesis,
TFEB
(as well as
MITF
and
TFE
3), is activated in an Atg5‐ and Atg9A‐dependent manner.
Similar articles
- N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.
Eldeeb MA, Ragheb MA. Eldeeb MA, et al. Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review. - The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications.
Durcan TM, Fon EA. Durcan TM, et al. Genes Dev. 2015 May 15;29(10):989-99. doi: 10.1101/gad.262758.115. Genes Dev. 2015. PMID: 25995186 Free PMC article. Review. - The Role of PTEN-L in Modulating PINK1-Parkin-Mediated Mitophagy.
Eldeeb MA, Esmaili M, Hassan M, Ragheb MA. Eldeeb MA, et al. Neurotox Res. 2022 Aug;40(4):1103-1114. doi: 10.1007/s12640-022-00475-w. Epub 2022 Jun 14. Neurotox Res. 2022. PMID: 35699891 Review. - miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1).
Kim J, Fiesel FC, Belmonte KC, Hudec R, Wang WX, Kim C, Nelson PT, Springer W, Kim J. Kim J, et al. Mol Neurodegener. 2016 Jul 26;11(1):55. doi: 10.1186/s13024-016-0121-4. Mol Neurodegener. 2016. PMID: 27456084 Free PMC article. - The mitochondrial kinase PINK1: functions beyond mitophagy.
Voigt A, Berlemann LA, Winklhofer KF. Voigt A, et al. J Neurochem. 2016 Oct;139 Suppl 1:232-239. doi: 10.1111/jnc.13655. Epub 2016 Jun 2. J Neurochem. 2016. PMID: 27251035 Review.
Cited by
- Anesthesia/surgery-induced learning and memory dysfunction by inhibiting mitophagy-mediated NLRP3 inflammasome inactivation in aged mice.
Lu J, Zong Y, Tao X, Dai H, Song J, Zhou H. Lu J, et al. Exp Brain Res. 2024 Feb;242(2):417-427. doi: 10.1007/s00221-023-06724-4. Epub 2023 Dec 26. Exp Brain Res. 2024. PMID: 38145993 Free PMC article. - Integrated proteogenetic analysis reveals the landscape of a mitochondrial-autophagosome synapse during PARK2-dependent mitophagy.
Heo JM, Harper NJ, Paulo JA, Li M, Xu Q, Coughlin M, Elledge SJ, Harper JW. Heo JM, et al. Sci Adv. 2019 Nov 6;5(11):eaay4624. doi: 10.1126/sciadv.aay4624. eCollection 2019 Nov. Sci Adv. 2019. PMID: 31723608 Free PMC article. - Loss and recovery of myocardial mitochondria in mice under different tail suspension time: Apoptosis and mitochondrial fission, fusion and autophagy.
Wang Z, Wang XC, Chen YF, Wang CL, Chen L, Jiang MY, Liu XW, Zhang XX, Feng YZ, Xu JH. Wang Z, et al. Exp Physiol. 2023 Sep;108(9):1189-1202. doi: 10.1113/EP090518. Epub 2023 Aug 11. Exp Physiol. 2023. PMID: 37565298 Free PMC article. - The Roles of SUMO in Metabolic Regulation.
Kamynina E, Stover PJ. Kamynina E, et al. Adv Exp Med Biol. 2017;963:143-168. doi: 10.1007/978-3-319-50044-7_9. Adv Exp Med Biol. 2017. PMID: 28197911 Free PMC article. Review. - Ubiquitin proteasome system in immune regulation and therapeutics.
Bhat SA, Vasi Z, Adhikari R, Gudur A, Ali A, Jiang L, Ferguson R, Liang D, Kuchay S. Bhat SA, et al. Curr Opin Pharmacol. 2022 Dec;67:102310. doi: 10.1016/j.coph.2022.102310. Epub 2022 Oct 23. Curr Opin Pharmacol. 2022. PMID: 36288660 Free PMC article. Review.
References
- Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39: 503–536 - PubMed
- Birkmayer W, Hornykiewicz O (1961) The L‐3,4‐dioxyphenylalanine (DOPA)‐effect in Parkinson‐akinesia. Wien Klin Wochenschr 73: 787–788 - PubMed
- Bras J, Guerreiro R, Hardy J (2015) SnapShot: genetics of Parkinson's disease. Cell 160: 570–570 e571 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous