Salmonella Suppresses the TRIF-Dependent Type I Interferon Response in Macrophages - PubMed (original) (raw)
Salmonella Suppresses the TRIF-Dependent Type I Interferon Response in Macrophages
Katherine A Owen et al. mBio. 2016.
Abstract
Salmonella enterica is an intracellular pathogen that causes diseases ranging from gastroenteritis to typhoid fever. Salmonella bacteria trigger an autophagic response in host cells upon infection but have evolved mechanisms for suppressing this response, thereby enhancing intracellular survival. We recently reported that S. enterica serovar Typhimurium actively recruits the host tyrosine kinase focal adhesion kinase (FAK) to the surface of the Salmonella-containing vacuole (SCV) (K. A. Owen et al., PLoS Pathog 10:e1004159, 2014). FAK then suppresses autophagy through activation of the Akt/mTORC1 signaling pathway. In FAK(-/-) macrophages, bacteria are captured in autophagosomes and intracellular survival is attenuated. Here we show that the cell-autonomous bacterial suppression of autophagy also suppresses the broader innate immune response by inhibiting production of beta interferon (IFN-β). Induction of bacterial autophagy (xenophagy), but not autophagy alone, triggers IFN-β production through a pathway involving the adapter TRIF and endosomal Toll-like receptor 3 (TLR3) and TLR4. Selective FAK knockout in macrophages resulted in rapid bacterial clearance from mucosal tissues after oral infection. Clearance correlated with increased IFN-β production by intestinal macrophages and with IFN-β-dependent induction of IFN-γ by intestinal NK cells. Blockade of either IFN-β or IFN-γ increased host susceptibility to infection, whereas experimental induction of IFN-β was protective. Thus, bacterial suppression of autophagy not only enhances cell-autonomous survival but also suppresses more-systemic innate immune responses by limiting type I and type II interferons.
Importance: Salmonella enterica serovar Typhimurium represents one of the most commonly identified bacterial causes of foodborne illness worldwide. S. Typhimurium has developed numerous strategies to evade detection by the host immune system. Autophagy is a cellular process that involves the recognition and degradation of defective proteins and organelles. More recently, autophagy has been described as an important means by which host cells recognize and eliminate invading intracellular pathogens and plays a key role in the production of cytokines. Previously, we determined that Salmonella bacteria are able to suppress their own autophagic capture and elimination by macrophages. Building on that study, we show here that the inhibition of autophagy by Salmonella also prevents the induction of a protective cytokine response mediated by beta interferon (IFN-β) and IFN-γ. Together, these findings identify a novel virulence strategy whereby Salmonella bacteria prevent cell autonomous elimination via autophagy and suppress the activation of innate immune responses.
Copyright © 2016 Owen et al.
Figures
FIGURE 1
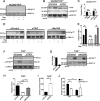
FAK deficiency in macrophages promotes IFN-β production in response to Salmonella infection. (A) Relative levels of expression of IFN-β mRNA by WT PEMs 5 h posttreatment with Salmonella LPS (100 ng/ml) or postinfection with the S. Typhimurium Δ_invG_ strain (MOI, 100). Levels of IFN-β mRNA are displayed relative to those seen with untreated/uninfected macrophages, which were assigned a value of 1 (see hashed line). *, P < 0.05; n = 5. (B) Relative levels of expression of IFN-β and IFN-α6 mRNA by WT and FAK−/− PEMs 5 h postinfection with the S. Typhimurium Δ_invG_ strain (MOI, 100). Levels of IFN-β and IFN-α6 mRNA are displayed relative to those seen with uninfected PEMs, which were assigned a value of 1 (horizontal dashed line). *, P < 0.05; n = 5. (C) WT and FAK−/− PEMs were incubated for 5 h with the S. Typhimurium Δ_invG_ strain before intracellular cytokine staining for IFN-β and analysis by flow cytometry. max, maximum. (D) The mean fluorescence intensities (MFIs) of cells analyzed as described for panel C were calculated and normalized to those of uninfected controls. Data are expressed as fold change compared to uninfected cells. *, P < 0.05; n = 3. (E) WT and FAK−/− PEMs were incubated for 0, 1, or 5 h with the S. Typhimurium Δ_invG_ strain before immunoblotting with the indicated antibodies. Separation of WT and FAK−/− immunoblots indicates noncontiguous lanes generated from a single exposure; n = 3. (F) FAK−/− PEMs were depleted of IRF-3 before immunoblotting with the indicated antibodies. Small interfering control (siCont.) and siIRF-3-treated FAK−/− macrophages were incubated for 5 h with the S. Typhimurium Δ_invG_ strain, and levels of IFN-β mRNA in infected cells were calculated relative to those seen with uninfected controls. *, P < 0.05; n = 3. Statistical significance was determined using a Student’s t test; error bars represent standard errors of the means (SEM). Densitometry was calculated using ImageJ.
FIGURE 2
Atg5 drives IFN-β production during Salmonella infection. (A) WT and FAK−/− PEMs were pretreated with rapamycin (1 nM) for 1 h or left untreated before incubation with the S. Typhimurium Δ_invG_ strain (MOI, 100) for 5 h. Lysates were immunoblotted with the indicated antibodies; n = 3. (B) WT PEMs were treated as described for panel A, and IFN-β mRNA levels in infected cells were calculated relative to those seen with uninfected controls. *, P < 0.05; n = 3. (C) FAK−/− PEMs were depleted of Atg5 before incubation with the S. Typhimurium Δ_invG_ strain for 5 h. Lysates were immunoblotted with the indicated antibodies; n = 3. (D and E) FAK−/− PEMs were either depleted of Atg5 (D) or treated with the autophagy inhibitor 3-MA (5 mM) (E) before incubation for 5 h with the S. Typhimurium Δ_invG_ strain. Levels of IFN-β mRNA in infected cells were calculated relative to those seen with uninfected controls. *, P < 0.05; n = 3. Statistical significance was determined using a Student’s t test; error bars represent SEM.
FIGURE 3
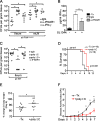
IFN-β production in response to Salmonella infection requires TRIF. (A and B) Immortalized macrophages (iMacs) deficient in both MyD88 and TRIF (MyD88−/−/TRIF−/−) were pretreated with rapamycin (1 nM) for 1 h (A) or were depleted of FAK by siRNA before incubation with Δ_invG Salmonella_ (B) (MOI, 100) for 5 h. Lysates were immunoblotted with the indicated antibodies; n = 3. (C) Macrophages were treated as described for panels A and B before assessment of IFN-β mRNA levels. IFN-β amounts in infected cells were calculated relative to those seen with uninfected controls. n.s., not significant; n = 3. (D) WT iMacs were depleted of TRIF or MyD88 before pretreatment with rapamycin (1 nM) for 1 h prior to infection with Δ_InvG Salmonella_ for 5 h. Lysates were immunoblotted with the indicated antibodies; n = 3. (E) Macrophages were treated as described for panel D before assessment of IFN-β mRNA levels. IFN-β amounts in infected cells were calculated relative to those seen with uninfected controls. *, P < 0.05; n.s., not significant; n = 3. (F and G) FAK−/− macrophages were depleted of TRIF or MyD88 before infection with Δ_invG Salmonella_ (MOI, 100) for 5 h. Lysates were then immunoblotted with the indicated antibodies; n = 3. In panel G, MyD88 knockdown efficiency was determined by assessing MyD88 mRNA levels relative to those seen with siCont.-treated cells. (H) FAK−/− macrophages were treated as described for panel F and G before measurement of IFN-β mRNA levels. IFN-β amounts in infected cells were calculated relative to those seen with uninfected controls. *, P < 0.05; n = 3. (I) FAK−/− macrophages depleted of TRIF were incubated with the S. Typhimurium Δ_invG_ strain for 3 h. Bacterial survival was assayed using a gentamicin resistance assay. (J) WT and FAK−/− macrophages were left untreated or treated with 5 µg/ml poly(I:C) before incubation with the Δ_invG_ strain for 3 h. Bacterial survival was then assayed as described for panel I. *, P < 0.05; n = 3. Statistical significance was determined using a Student’s t test; error bars represent SEM.
FIGURE 4
TLR3 and TLR4 are involved in IFN-β production by macrophages in response to Salmonella infection. (A and B) iMacs derived from TLR4-deficient mice (TLR4−/−) were pretreated for 1 h with rapamycin (1 nM) (A) or depleted of FAK (B) before infection with Δ_invG Salmonella_ (MOI, 100) for 5 h. Lysates were then immunoblotted with the indicated antibodies; n = 3. (C) IFN-β mRNA levels in cells treated as described for panels A and B were calculated relative to those seen with uninfected controls. n.s., not significant; n = 3. (D) WT iMacs depleted of TLR3 were pretreated for 1 h with rapamycin (1 nM) before infection with Δ_invG Salmonella_ (MOI, 100) for 5 h. Lysates were then immunoblotted with the indicated antibodies; n = 3. TLR3 knockdown efficiency was determined by assessing TLR3 mRNA levels relative to those seen with siCont.-treated cells. (E) IFN-β mRNA levels in cells treated as described for panel D were calculated relative to those seen with uninfected controls. *, P < 0.05; n = 3. (F) FAK−/− macrophages were depleted of TLR3 before infection with Δ_invG Salmonella_ (MOI, 100) for 5 h. Lysates were then immunoblotted with the indicated antibodies; n = 3. (G) FAK−/− macrophages were either treated as described for panel F or pretreated with bafilomycin A1 (Baf.A1) (100 nM) for 1 h before infection for 5 h with the S. Typhimurium Δ_invG_ strain. IFN-β mRNA levels in infected cells were calculated relative to those seen with uninfected controls. *, P < 0.05; n = 3. Statistical significance was determined using a Student’s t test; error bars represent SEM. (H) Model showing that autophagic conditions (FAK deletion or sirolimus treatment) promote the delivery of antigenic material to TLR-containing endosomes, resulting in IFN-β upregulation. Inhibition at each step (step 1, autophagosomes; step 2, autolysosomes; steps 3 and 4, TLR signaling; step 5, TRIF/IRF3 signaling) suppresses IFN-β. PM, plasma membrane; SCV, _Salmonella_-containing vacuole; Ly, lysosome.
FIGURE 5
FAK deficiency improves control of Salmonella infection and enhances the IFN response in vivo. (A) Bacterial loads in WT and FAKΔmyeloid mice in the indicated tissues 2 days after oral inoculation with S. Typhimurium strain SL1344. Each point indicates data from an individual mouse. *, P < 0.05 (Mann-Whitney U test). (B) Flow cytometric analysis of IFN-β-expressing cells in the lamina propria (LP) (taken from WT and FAKΔmyeloid mice 2 days [2d] postinfection [see also Fig. S4A in the supplemental material]). The IFN-β-positive population was further analyzed for expression of CD11c and F4-80. Numbers adjacent to each gate indicate percentages of cells in the gated region. Data represent results of one of two independent experiments performed using cells pooled from three mice (6 mice total). Fsc-L, forward-scatter light. (C) Lamina propria lysates from naive and infected (2 days) WT and FAKΔmyeloid mice were immunoblotted for phospho–IRF-3. Data represent results of one of three independent experiments. (D) IFN-γ levels were analyzed in LP lysates extracted from naive or infected (2 days) WT and FAKΔmyeloid mice by Luminex assay. Data are expressed as fold increase in postinfection IFN-γ levels relative to those seen with naive mice. Data represent results of 3 independent experiments using LP lysates pooled from 3 mice per condition and per experiment. *, P < 0.05. (E) Flow cytometric analysis of IFN-γ-expressing cells in the LP (taken from WT and FAKΔmyeloid mice 2 days postinfection [see also Fig. S4B]). The IFN-γ-positive population was further analyzed for expression of CD3ε and NK1.1 to identify NK cells. Numbers adjacent to each gate indicate percentages of cells in the gated region. Data are representative of results of one of three independent experiments using cells pooled from 3 mice (9 mice total). (F) Quantitation of IFN-γ-positive cells based on the flow cytometric data as described for panel B. *, P < 0.05. See also Table S1. (G) LP lysates from naive and infected (2 days) WT and FAKΔmyeloid mice were immunoblotted with the indicated antibodies. Immunoblots represent results of one of three independent experiments. Densitometry was calculated using ImageJ. Unless indicated otherwise, statistical significance was determined using a Student’s t test; error bars represent SEM.
FIGURE 6
The induction of IFN-β induces protective immunity in intestinal tissues. (A) FAKΔmyeloid mice were treated with control IgG, blocking anti-IFNAR1 antibodies (250 µg/mouse), or neutralizing anti-IFN-γ antibodies (250 µg/mouse) 1 day prior to infection with S. Typhimurium strain SL1344. Bacterial loads were assessed in the indicated tissues 2 days postinfection. Each point indicates data from an individual mouse. *, P < 0.05 (Mann-Whitney U test). (B) IFN-γ levels were analyzed in LP lysates extracted from FAKΔmyeloid mice by Luminex assay. Animals were either left uninfected and untreated (-Tx) or treated with control IgG or anti-IFNAR1 antibodies (250 µg/mouse) 1 day prior to infection with SL1344 (2 days). Data represent LP lysates from 3 independent experiments. *, P < 0.05. (C) WT mice were treated with control IgG, poly(I:C) (100 µg/mouse), or poly(I:C) in combination with blocking anti-IFNAR1 antibodies (250 µg/mouse) 1 day prior to infection with S. Typhimurium strain SL1344. Bacterial loads were assessed in the ileum 2 days postinfection. Each point indicates data from an individual mouse. *, P < 0.05 (Mann-Whitney U test). (D) Kaplan-Meier survival curve for WT mice that were left untreated (-Tx) or administered poly(I:C) 1 day prior to infection with 108 CFU/mouse of S. Typhimurium strain SL1344. A total of 10 mice per condition were analyzed. (E) WT mice were treated as described for panel D. Percent change in body weight was calculated on day 6. Each point indicates data from an individual mouse. *, P < 0.05. (F) WT mice treated as described for panel D were assessed daily for disease activity (see Materials and Methods). Data were pooled from results of 2 independent experiments. n = 10; *, P < 0.05. Unless indicated otherwise, statistical significance was determined using a Student’s t test; error bars represent SEM.
FIGURE 7
FAKΔmyeloid animals exhibit an accelerated innate immune response to Salmonella infection. (A) mLNs from WT and FAKΔmyeloid mice infected with SL1344 for 2 days were isolated and analyzed for IFN-γ, CXCL9, and CXCL10 by Luminex assay. Data are expressed as fold change relative to the WT and represent results of 3 independent experiments using mLN lysates pooled from 3 mice per experiment. *, P < 0.05. (B) Panels show expression of Ly6G and Ly6C in live CD45hi SSChi cells from the mLNs of WT and FAKΔmyeloid mice at 2 days postinfection with SL1344. Gate R1 indicates neutrophils (Ly6Ghi CD11bhi Ly6C+), and gate R2 indicates inflammatory monocytes (Ly6Chi CD11b+). Numbers adjacent to each gate indicate percentages of cells in R1 or R2. Data are representative of results of one of three independent experiments using cells pooled from 3 mice per experiment (see also Fig. S4C in the supplemental material). (C) Quantitation of flow cytometric data (as described for panel B) collected from naive and infected mice. *, P < 0.05. (D) WT and FAKΔmyeloid mice were treated with control IgG or neutralizing anti-Gr1 antibodies (250 µg/mouse) 1 day prior to infection with S. Typhimurium strain SL1344. Bacterial loads were assessed in the mLNs 2 days postinfection. Each point indicates data from an individual mouse. *, P < 0.05 (Mann-Whitney U test). (E) Integrated working model describing the cell intrinsic and extrinsic mechanism of bacterial killing in autophagic macrophages. In WT macrophages (depicted in panel a), Salmonella bacteria activate the FAK-Akt-mTOR signaling pathway to suppress autophagy. Intracellular survival is enhanced, and in the absence of an IFN response, bacteria disseminate. In autophagic macrophages (panel b), Salmonella bacteria are captured and degraded in autophagosomes. Autophagy promotes the delivery of antigenic material to TLR-containing endosomes (see also Fig. 4H), which activates the TRIF–IRF-3 pathway to induce the production of IFN-β. IFN-β activates NK cells and promotes the production of IFN-γ. The presence of IFN-γ is able to activate additional phagocytes as well as stimulate the production of CXC chemokines. CXCL9 and CXCL10 help recruit neutrophils to the site of infection and further prevent the dissemination of bacteria.
Similar articles
- Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages.
Owen KA, Meyer CB, Bouton AH, Casanova JE. Owen KA, et al. PLoS Pathog. 2014 Jun 5;10(6):e1004159. doi: 10.1371/journal.ppat.1004159. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24901456 Free PMC article. - Host innate recognition of an intestinal bacterial pathogen induces TRIF-dependent protective immunity.
Sotolongo J, España C, Echeverry A, Siefker D, Altman N, Zaias J, Santaolalla R, Ruiz J, Schesser K, Adkins B, Fukata M. Sotolongo J, et al. J Exp Med. 2011 Dec 19;208(13):2705-16. doi: 10.1084/jem.20110547. Epub 2011 Nov 28. J Exp Med. 2011. PMID: 22124111 Free PMC article. - Toll-Like receptor 2 (TLR2) and TLR9 play opposing roles in host innate immunity against Salmonella enterica serovar Typhimurium infection.
Zhan R, Han Q, Zhang C, Tian Z, Zhang J. Zhan R, et al. Infect Immun. 2015 Apr;83(4):1641-9. doi: 10.1128/IAI.02870-14. Epub 2015 Feb 9. Infect Immun. 2015. PMID: 25667264 Free PMC article. - Bacterial Autophagy: Offense and Defense at the Host-Pathogen Interface.
Casanova JE. Casanova JE. Cell Mol Gastroenterol Hepatol. 2017 May 6;4(2):237-243. doi: 10.1016/j.jcmgh.2017.05.002. eCollection 2017 Sep. Cell Mol Gastroenterol Hepatol. 2017. PMID: 28660242 Free PMC article. Review. - Host-pathogen interaction in invasive Salmonellosis.
de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. de Jong HK, et al. PLoS Pathog. 2012;8(10):e1002933. doi: 10.1371/journal.ppat.1002933. Epub 2012 Oct 4. PLoS Pathog. 2012. PMID: 23055923 Free PMC article. Review.
Cited by
- Beyond the List: Bioagent-Agnostic Signatures Could Enable a More Flexible and Resilient Biodefense Posture Than an Approach Based on Priority Agent Lists Alone.
Leiser OP, Hobbs EC, Sims AC, Korch GW, Taylor KL. Leiser OP, et al. Pathogens. 2021 Nov 17;10(11):1497. doi: 10.3390/pathogens10111497. Pathogens. 2021. PMID: 34832652 Free PMC article. - Impact of Type I Interferons on Susceptibility to Bacterial Pathogens.
Peignier A, Parker D. Peignier A, et al. Trends Microbiol. 2021 Sep;29(9):823-835. doi: 10.1016/j.tim.2021.01.007. Epub 2021 Feb 2. Trends Microbiol. 2021. PMID: 33546974 Free PMC article. Review. - Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release.
Xu L, Li M, Yang Y, Zhang C, Xie Z, Tang J, Shi Z, Chen S, Li G, Gu Y, Wang X, Zhang F, Wang Y, Shen X. Xu L, et al. mBio. 2022 Jun 28;13(3):e0363221. doi: 10.1128/mbio.03632-21. Epub 2022 May 23. mBio. 2022. PMID: 35604097 Free PMC article. - Mycoplasma hyorhinis reduces sensitivity of human lung carcinoma cells to Nutlin-3 and promotes their malignant phenotype.
Boyarskikh UA, Shadrina AS, Smetanina MA, Tsepilov YA, Oscorbin IP, Kozlov VV, Kel AE, Filipenko ML. Boyarskikh UA, et al. J Cancer Res Clin Oncol. 2018 Jul;144(7):1289-1300. doi: 10.1007/s00432-018-2658-9. Epub 2018 May 8. J Cancer Res Clin Oncol. 2018. PMID: 29737431
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK058536/DK/NIDDK NIH HHS/United States
- 5F32DK839022/DK/NIDDK NIH HHS/United States
- T32 AI007046/AI/NIAID NIH HHS/United States
- R01DK58536/DK/NIDDK NIH HHS/United States
- T32 AI055432/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous