Pioglitazone after Ischemic Stroke or Transient Ischemic Attack - PubMed (original) (raw)
Randomized Controlled Trial
. 2016 Apr 7;374(14):1321-31.
doi: 10.1056/NEJMoa1506930. Epub 2016 Feb 17.
Catherine M Viscoli 1, Karen L Furie 1, Lawrence H Young 1, Silvio E Inzucchi 1, Mark Gorman 1, Peter D Guarino 1, Anne M Lovejoy 1, Peter N Peduzzi 1, Robin Conwit 1, Lawrence M Brass 1, Gregory G Schwartz 1, Harold P Adams Jr 1, Leo Berger 1, Antonio Carolei 1, Wayne Clark 1, Bruce Coull 1, Gary A Ford 1, Dawn Kleindorfer 1, John R O'Leary 1, Mark W Parsons 1, Peter Ringleb 1, Souvik Sen 1, J David Spence 1, David Tanne 1, David Wang 1, Toni R Winder 1; IRIS Trial Investigators
Collaborators, Affiliations
- PMID: 26886418
- PMCID: PMC4887756
- DOI: 10.1056/NEJMoa1506930
Randomized Controlled Trial
Pioglitazone after Ischemic Stroke or Transient Ischemic Attack
Walter N Kernan et al. N Engl J Med. 2016.
Abstract
Background: Patients with ischemic stroke or transient ischemic attack (TIA) are at increased risk for future cardiovascular events despite current preventive therapies. The identification of insulin resistance as a risk factor for stroke and myocardial infarction raised the possibility that pioglitazone, which improves insulin sensitivity, might benefit patients with cerebrovascular disease.
Methods: In this multicenter, double-blind trial, we randomly assigned 3876 patients who had had a recent ischemic stroke or TIA to receive either pioglitazone (target dose, 45 mg daily) or placebo. Eligible patients did not have diabetes but were found to have insulin resistance on the basis of a score of more than 3.0 on the homeostasis model assessment of insulin resistance (HOMA-IR) index. The primary outcome was fatal or nonfatal stroke or myocardial infarction.
Results: By 4.8 years, a primary outcome had occurred in 175 of 1939 patients (9.0%) in the pioglitazone group and in 228 of 1937 (11.8%) in the placebo group (hazard ratio in the pioglitazone group, 0.76; 95% confidence interval [CI], 0.62 to 0.93; P=0.007). Diabetes developed in 73 patients (3.8%) and 149 patients (7.7%), respectively (hazard ratio, 0.48; 95% CI, 0.33 to 0.69; P<0.001). There was no significant between-group difference in all-cause mortality (hazard ratio, 0.93; 95% CI, 0.73 to 1.17; P=0.52). Pioglitazone was associated with a greater frequency of weight gain exceeding 4.5 kg than was placebo (52.2% vs. 33.7%, P<0.001), edema (35.6% vs. 24.9%, P<0.001), and bone fracture requiring surgery or hospitalization (5.1% vs. 3.2%, P=0.003).
Conclusions: In this trial involving patients without diabetes who had insulin resistance along with a recent history of ischemic stroke or TIA, the risk of stroke or myocardial infarction was lower among patients who received pioglitazone than among those who received placebo. Pioglitazone was also associated with a lower risk of diabetes but with higher risks of weight gain, edema, and fracture. (Funded by the National Institute of Neurological Disorders and Stroke; ClinicalTrials.gov number, NCT00091949.).
Figures
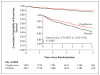
Figure 1. Primary Outcome
By 5 years, the primary outcome (fatal or nonfatal stroke or fatal or nonfatal myocardial infarction) had occurred in 175 of 1939 patients (9.0%) in the pioglitazone group and in 228 of 1937 (11.8%) in the placebo group. The inset shows the same data on an enlarged y axis. The numbers at risk were the numbers of patients who were alive without an event and still being followed at the beginning of each time point.
Figure 2. Subgroup Analyses of the Primary Outcome
Shown is the relative benefit of pioglitazone as compared with placebo in 13 subgroups that were examined for their interaction with the treatment. P values have not been adjusted for multiple comparisons. (P = 0.94 after adjustment for multiple comparisons in each subgroup.) The size of the squares corresponds to the number of patients in each subgroup. The body-mass index is the weight in kilograms divided by the square of the height in meters. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for glucose to millimoles per liter, multiply by 0.05551. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. HDL denotes high-density lipoprotein, and HOMA-IR homeostasis model assessment of insulin resistance.
Comment in
- Insulin Resistance and a Long, Strange Trip.
Semenkovich CF. Semenkovich CF. N Engl J Med. 2016 Apr 7;374(14):1378-9. doi: 10.1056/NEJMe1600962. Epub 2016 Feb 17. N Engl J Med. 2016. PMID: 26886417 No abstract available. - Pioglitazone after Ischemic Stroke or Transient Ischemic Attack.
Viscoli CM, Kernan WN, Young LH. Viscoli CM, et al. N Engl J Med. 2016 Aug 18;375(7):704. doi: 10.1056/NEJMc1605904. N Engl J Med. 2016. PMID: 27532845 No abstract available. - Pioglitazone after Ischemic Stroke or Transient Ischemic Attack.
Bursztyn M. Bursztyn M. N Engl J Med. 2016 Aug 18;375(7):702-3. doi: 10.1056/NEJMc1605904. N Engl J Med. 2016. PMID: 27532846 No abstract available. - Pioglitazone after Ischemic Stroke or Transient Ischemic Attack.
Doehner W, Anker SD. Doehner W, et al. N Engl J Med. 2016 Aug 18;375(7):703. doi: 10.1056/NEJMc1605904. N Engl J Med. 2016. PMID: 27532847 No abstract available. - Pioglitazone after Ischemic Stroke or Transient Ischemic Attack.
Hu K, Xu W, Williams Z. Hu K, et al. N Engl J Med. 2016 Aug 18;375(7):703-4. doi: 10.1056/NEJMc1605904. N Engl J Med. 2016. PMID: 27532848 No abstract available. - Pioglitazone in patients with insulin resistance after ischemic stroke or transient ischemic attack: A comment on the IRIS trial.
Katsiki N, Mikhailidis DP. Katsiki N, et al. J Diabetes Complications. 2017 Jan;31(1):1-3. doi: 10.1016/j.jdiacomp.2016.09.005. Epub 2016 Sep 18. J Diabetes Complications. 2017. PMID: 28340963 No abstract available.
Similar articles
- Targeting Pioglitazone Hydrochloride Therapy After Stroke or Transient Ischemic Attack According to Pretreatment Risk for Stroke or Myocardial Infarction.
Kernan WN, Viscoli CM, Dearborn JL, Kent DM, Conwit R, Fayad P, Furie KL, Gorman M, Guarino PD, Inzucchi SE, Stuart A, Young LH; Insulin Resistance Intervention After Stroke (IRIS) Trial Investigators. Kernan WN, et al. JAMA Neurol. 2017 Nov 1;74(11):1319-1327. doi: 10.1001/jamaneurol.2017.2136. JAMA Neurol. 2017. PMID: 28975241 Free PMC article. Clinical Trial. - Pioglitazone Prevents Diabetes in Patients With Insulin Resistance and Cerebrovascular Disease.
Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, Dagogo-Jack S, Ismail-Beigi F, Korytkowski MT, Pratley RE, Schwartz GG, Kernan WN; IRIS Trial Investigators. Inzucchi SE, et al. Diabetes Care. 2016 Oct;39(10):1684-92. doi: 10.2337/dc16-0798. Epub 2016 Jul 27. Diabetes Care. 2016. PMID: 27465265 Free PMC article. Clinical Trial. - Heart Failure After Ischemic Stroke or Transient Ischemic Attack in Insulin-Resistant Patients Without Diabetes Mellitus Treated With Pioglitazone.
Young LH, Viscoli CM, Schwartz GG, Inzucchi SE, Curtis JP, Gorman MJ, Furie KL, Conwit R, Spatz ES, Lovejoy A, Abbott JD, Jacoby DL, Kolansky DM, Ling FS, Pfau SE, Kernan WN; IRIS Investigators. Young LH, et al. Circulation. 2018 Sep 18;138(12):1210-1220. doi: 10.1161/CIRCULATIONAHA.118.034763. Circulation. 2018. PMID: 29934374 Free PMC article. Clinical Trial. - Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack.
Liu J, Wang LN. Liu J, et al. Cochrane Database Syst Rev. 2015 Oct 29;(10):CD010693. doi: 10.1002/14651858.CD010693.pub3. Cochrane Database Syst Rev. 2015. PMID: 26511368 Updated. Review. - Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack.
Liu J, Wang LN. Liu J, et al. Cochrane Database Syst Rev. 2014 Jan 8;(1):CD010693. doi: 10.1002/14651858.CD010693.pub2. Cochrane Database Syst Rev. 2014. PMID: 24399670 Updated. Review.
Cited by
- Ponatinib and other CML Tyrosine Kinase Inhibitors in Thrombosis.
Zeng P, Schmaier A. Zeng P, et al. Int J Mol Sci. 2020 Sep 8;21(18):6556. doi: 10.3390/ijms21186556. Int J Mol Sci. 2020. PMID: 32911643 Free PMC article. Review. - Pharmacological Approaches to Nonalcoholic Fatty Liver Disease: Current and Future Therapies.
Genua I, Cusi K. Genua I, et al. Diabetes Spectr. 2024 Winter;37(1):48-58. doi: 10.2337/dsi23-0012. Epub 2024 Feb 15. Diabetes Spectr. 2024. PMID: 38385098 Free PMC article. - Anti-inflammatory and anti-proliferative action of adiponectin mediated by insulin signaling cascade in human vascular smooth muscle cells.
Cersosimo E, Xu X, Terasawa T, Dong LQ. Cersosimo E, et al. Mol Biol Rep. 2020 Sep;47(9):6561-6572. doi: 10.1007/s11033-020-05707-w. Epub 2020 Aug 12. Mol Biol Rep. 2020. PMID: 32789574 - Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes.
Lisco G, De Tullio A, Iovino M, Disoteo O, Guastamacchia E, Giagulli VA, Triggiani V. Lisco G, et al. Biomedicines. 2023 Nov 7;11(11):2993. doi: 10.3390/biomedicines11112993. Biomedicines. 2023. PMID: 38001993 Free PMC article. Review. - Thiazolidinediones: the Forgotten Diabetes Medications.
Lebovitz HE. Lebovitz HE. Curr Diab Rep. 2019 Nov 27;19(12):151. doi: 10.1007/s11892-019-1270-y. Curr Diab Rep. 2019. PMID: 31776781 Free PMC article. Review.
References
- Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–3. - PubMed
- Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. 2006;66:641–6. - PubMed
- Johnston SC. Transient ischemic attack. N Engl J Med. 2002;347:1687–92. - PubMed
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01NS044876/NS/NINDS NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- U01 NS044876/NS/NINDS NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical