Energetics of Transport through the Nuclear Pore Complex - PubMed (original) (raw)
Energetics of Transport through the Nuclear Pore Complex
Ali Ghavami et al. PLoS One. 2016.
Abstract
Molecular transport across the nuclear envelope in eukaryotic cells is solely controlled by the nuclear pore complex (NPC). The NPC provides two types of nucleocytoplasmic transport: passive diffusion of small molecules and active chaperon-mediated translocation of large molecules. It has been shown that the interaction between intrinsically disordered proteins that line the central channel of the NPC and the transporting cargoes is the determining factor, but the exact mechanism of transport is yet unknown. Here, we use coarse-grained molecular dynamics simulations to quantify the energy barrier that has to be overcome for molecules to pass through the NPC. We focus on two aspects of transport. First, the passive transport of model cargo molecules with different sizes is studied and the size selectivity feature of the NPC is investigated. Our results show that the transport probability of cargoes is significantly reduced when they are larger than ∼5 nm in diameter. Secondly, we show that incorporating hydrophobic binding spots on the surface of the cargo effectively decreases the energy barrier of the pore. Finally, a simple transport model is proposed which characterizes the energy barrier of the NPC as a function of diameter and hydrophobicity of the transporting particles.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Fig 1. The geometrical model of the NPC and cargoes.
(A) (left) The core scaffold of the NPC, reconstructed based on the structural model proposed in [45], (right) a snapshot taken from an umbrella sampling simulation for a cargo with D = 10 nm. (B) Geometrical representation of a model cargo smaller than 5.0 nm in diameter and (C), composite cargoes larger than 5.0 nm. (D) Geometrical representation of a model Kap-cargo complex with 7 binding spots. (E) The inert cargoes with different diameters and a Kap-cargo complex as used in the simulations.
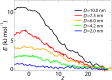
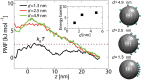
Fig 2. Potential of mean force curves along the central axis of the NPC (r = 0) for cargoes with D = 10, 7.3, 6.0, 4.2 and 2.0 nm.
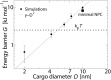
Fig 3. The energy barrier G versus diameter D of the cargoes.
The dashed line is a quadratic fit to the data and the error bars indicate the standard deviation of the data for the interval -5.0 nm < z < 5.0 nm and 20 nm < z <27 nm.
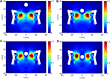
Fig 4. The 2D density plots of the FG-Nups taken from the umbrella simulations.
The vertical distance from the center of the cargo to the central plane of the NPC is (A) 30 nm, (B) 15.6 nm, (C) 8.3 and (D) 1.1 nm, respectively.
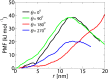
Fig 5. The potential of mean force along the radial direction of the NPC (at z = −2.5 nm) for a cargo with D = 10 nm.
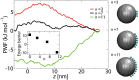
Fig 6. The free energy curves along the central axis of the NPC (r = 0), for a Kap-cargo complex of diameter D = 10 nm with different spacings d between the binding spots on its surface.
The inset shows the energy barrier versus spacing d. The interaction energy between individual binding spots and the FG-repeats is −5.2 kJ.mol−1.
Fig 7. The free energy curves along the central axis of the NPC (r = 0), for a Kap-cargo complex of diameter D = 10 nm with different number of binding spots n.
The inset shows the energy barrier versus the number of binding spots d. The interaction energy between individual binding spots and the FG-repeats is −5.2 kJ.mol−1.
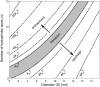
Fig 8. Contour plot of the energy barrier G of the NPC as a function of cargo diameter D and number of hydrophobic binding spots n.
Similar articles
- Molecular determinants of large cargo transport into the nucleus.
Paci G, Zheng T, Caria J, Zilman A, Lemke EA. Paci G, et al. Elife. 2020 Jul 21;9:e55963. doi: 10.7554/eLife.55963. Elife. 2020. PMID: 32692309 Free PMC article. - Introduction to nucleocytoplasmic transport: molecules and mechanisms.
Peters R. Peters R. Methods Mol Biol. 2006;322:235-58. doi: 10.1007/978-1-59745-000-3_17. Methods Mol Biol. 2006. PMID: 16739728 Review. - Nucleoporin's Like Charge Regions Are Major Regulators of FG Coverage and Dynamics Inside the Nuclear Pore Complex.
Peyro M, Soheilypour M, Ghavami A, Mofrad MR. Peyro M, et al. PLoS One. 2015 Dec 11;10(12):e0143745. doi: 10.1371/journal.pone.0143745. eCollection 2015. PLoS One. 2015. PMID: 26658558 Free PMC article. - Efficiency, selectivity, and robustness of nucleocytoplasmic transport.
Zilman A, Di Talia S, Chait BT, Rout MP, Magnasco MO. Zilman A, et al. PLoS Comput Biol. 2007 Jul;3(7):e125. doi: 10.1371/journal.pcbi.0030125. PLoS Comput Biol. 2007. PMID: 17630825 Free PMC article. - Biomechanics of the transport barrier in the nuclear pore complex.
Stanley GJ, Fassati A, Hoogenboom BW. Stanley GJ, et al. Semin Cell Dev Biol. 2017 Aug;68:42-51. doi: 10.1016/j.semcdb.2017.05.007. Epub 2017 May 12. Semin Cell Dev Biol. 2017. PMID: 28506890 Review.
Cited by
- A designer FG-Nup that reconstitutes the selective transport barrier of the nuclear pore complex.
Fragasso A, de Vries HW, Andersson J, van der Sluis EO, van der Giessen E, Dahlin A, Onck PR, Dekker C. Fragasso A, et al. Nat Commun. 2021 Mar 31;12(1):2010. doi: 10.1038/s41467-021-22293-y. Nat Commun. 2021. PMID: 33790297 Free PMC article. - Molecular determinants of large cargo transport into the nucleus.
Paci G, Zheng T, Caria J, Zilman A, Lemke EA. Paci G, et al. Elife. 2020 Jul 21;9:e55963. doi: 10.7554/eLife.55963. Elife. 2020. PMID: 32692309 Free PMC article. - Single-nucleus proteomics identifies regulators of protein transport.
Derks J, Jonson T, Leduc A, Khan S, Khoury L, Rafiee MR, Slavov N. Derks J, et al. bioRxiv [Preprint]. 2024 Jun 18:2024.06.17.599449. doi: 10.1101/2024.06.17.599449. bioRxiv. 2024. PMID: 38948785 Free PMC article. Preprint. - Protein Transport by the Nuclear Pore Complex: Simple Biophysics of a Complex Biomachine.
Jovanovic-Talisman T, Zilman A. Jovanovic-Talisman T, et al. Biophys J. 2017 Jul 11;113(1):6-14. doi: 10.1016/j.bpj.2017.05.024. Biophys J. 2017. PMID: 28700925 Free PMC article. Review. - Physical modeling of multivalent interactions in the nuclear pore complex.
Davis LK, Šarić A, Hoogenboom BW, Zilman A. Davis LK, et al. Biophys J. 2021 May 4;120(9):1565-1577. doi: 10.1016/j.bpj.2021.01.039. Epub 2021 Feb 20. Biophys J. 2021. PMID: 33617830 Free PMC article.
References
- Feldherr C, Akin D. The location of the transport gate in the nuclear pore complex. Journal of Cell Science. 1997;110(24):3065 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This research was funded by the Zernike Institute for Advanced Materials at the University of Groningen. The authors thank SURFsara (www.surfsara.nl) for the support in using the Lisa Compute Cluster. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources