Endothelial Plasticity: Shifting Phenotypes through Force Feedback - PubMed (original) (raw)
Review
Endothelial Plasticity: Shifting Phenotypes through Force Feedback
Guido Krenning et al. Stem Cells Int. 2016.
Abstract
The endothelial lining of the vasculature is exposed to a large variety of biochemical and hemodynamic stimuli with different gradients throughout the vascular network. Adequate adaptation requires endothelial cells to be highly plastic, which is reflected by the remarkable heterogeneity of endothelial cells in tissues and organs. Hemodynamic forces such as fluid shear stress and cyclic strain are strong modulators of the endothelial phenotype and function. Although endothelial plasticity is essential during development and adult physiology, proatherogenic stimuli can induce adverse plasticity which contributes to disease. Endothelial-to-mesenchymal transition (EndMT), the hallmark of endothelial plasticity, was long thought to be restricted to embryonic development but has emerged as a pathologic process in a plethora of diseases. In this perspective we argue how shear stress and cyclic strain can modulate EndMT and discuss how this is reflected in atherosclerosis and pulmonary arterial hypertension.
Figures
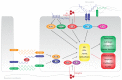
Figure 1
Mechanisms of endothelial-mesenchymal transition. EndMT is induced by TGF_β_-dependent and TGF_β_-independent mechanisms. Canonically, TGF_β_-induced activation of SMAD2/3 induces the expression of mesenchymal genes and repression of endothelial genes. Noncanonically, TGF_β_-induced activation of Erk1/2 and p38 MAPK activate the transcription factor Snail which induces mesenchymal differentiation and inhibits VE-Cadherin expression. Inflammatory signals and increased ROS production facilitate EndMT by increasing endogenous TGF_β_ expression, in an NF_κ_B-dependent manner, creating a feed forward signaling mechanism. AT1 receptor can induce EndMT through activation of NADPH oxidase, resulting in increased ROS production and reduction of eNOS expression and activity.
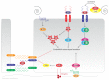
Figure 2
Endothelial shear stress sensing. Endothelial cells sense LSS through a number of mechanisms. Shear stress sensing through the endothelial glycocalyx is mediated by the syndecans and glypicans, which activate PKC signaling. Luminal β_1-integrins also anchor to the endothelial glycocalyx and activate focal adhesion kinase (FAK). The G-protein-coupled receptors of G_α q/G_α_ 11 sense shear stress and activate downstream PI3K and Ca2+ signaling. The junctional mechanosensory complex consisting of PECAM-1, VEGFR2, and VE-Cadherin mediates PI3K and MAPK (MEKK3) signaling. Signaling through these mechanosensors culminates in activation of Akt, PKA, AMPK, and MEK5/Erk5 which collectively maintain the endothelial phenotype.
Figure 3
Endothelial cyclic strain sensing. Endothelial cells sense CS through a number of mechanisms. Stretch-sensitive ion channels undergo a conformational change during stretch which opens up the channel and induces Ca2+ flux from the extracellular space into the endothelial cell. Basal β_1-integrins anchor to the endothelial basement membrane and activate focal adhesion kinase (FAK) and the integrin-linked kinases (ILK). The G-protein-coupled receptors of G_α q/G_α_ 11 sense CS and activate downstream PI3K and Ca2+ signaling. Signaling through these stretch sensors culminates in activation of Akt, PKC, PLC, and Erk1/2 which collectively maintain the endothelial phenotype.
Figure 4
Disturbed shear stress and high cyclic strain signaling in endothelial-mesenchymal transition. Disturbed shear stress induces EndMT through several mechanisms. Disturbed shear stress activates latent TGF_β_ by liberating it from LAP, after which TGF_β_ can induce Smad2/3 signaling. Disturbed shear stress induces the expression of BMP4 which causes ROS formation and the activity of NF_κ_B. Lastly, disturbed shear stress induces NOX and XO activity resulting in the generation of ROS. All these signaling intermediates culminate in EndMT. Increased cyclic strain (>10%) induces the FAK-dependent activation of Rho-kinases. Rho activity causes the translocation of VE-Cadherin from the cell membrane into cytoplasmic vesicles and causes a reduction in endothelial cell-cell contacts. Cyclic strain-dependent Wnt-_β_-catenin activity induces EndMT in part by the induction of Snail and Slug and the further activation of Rho activity.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources