Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation - PubMed (original) (raw)
. 2016 May;37(5):1941-52.
doi: 10.1002/hbm.23148. Epub 2016 Feb 25.
Affiliations
- PMID: 26915535
- PMCID: PMC6867525
- DOI: 10.1002/hbm.23148
Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation
Milan Scheidegger et al. Hum Brain Mapp. 2016 May.
Abstract
Increased amygdala reactivity might lead to negative bias during emotional processing that can be reversed by antidepressant drug treatment. However, little is known on how N-methyl-d-aspartate (NMDA) receptor antagonism with ketamine as a novel antidepressant drug target might modulate amygdala reactivity to emotional stimulation. Using functional magnetic resonance imaging (fMRI) and resting-state fMRI (rsfMRI), we assessed amygdalo-hippocampal reactivity at baseline and during pharmacological stimulation with ketamine (intravenous bolus of 0.12 mg/kg, followed by a continuous infusion of 0.25 mg/kg/h) in 23 healthy subjects that were presented with stimuli from the International Affective Picture System (IAPS). We found that ketamine reduced neural reactivity in the bilateral amygdalo-hippocampal complex during emotional stimulation. Reduced amygdala reactivity to negative pictures was correlated to resting-state connectivity to the pregenual anterior cingulate cortex. Interestingly, subjects experienced intensity of psychedelic alterations of consciousness during ketamine infusion predicted the reduction in neural responsivity to negative but not to positive or neutral stimuli. Our findings suggest that the pharmacological modulation of glutamate-responsive cerebral circuits, which is associated with a shift in emotional bias and a reduction of amygdalo-hippocampal reactivity to emotional stimuli, represents an early biomechanism to restore parts of the disrupted neurobehavioral homeostasis in MDD patients. Hum Brain Mapp 37:1941-1952, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: antidepressants; depression; emotional processing; fMRI; glutamate; ketamine.
© 2016 Wiley Periodicals, Inc.
Figures
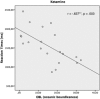
Figure 1
Significant negative correlation between reaction times and increased scores in the scales of oceanic boundlessness in the ketamine condition (n = 20, r = −0.637, p = 0.003).
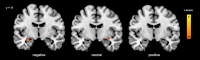
Figure 2
Reduced BOLD reactivity in the amygdalo‐hippocampal complex during processing of positive, negative, and neutral stimuli after ketamine administration compared to baseline (whole brain paired t test, n = 23, FDR‐corrected, k > 25).
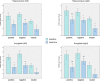
Figure 3
Reduced BOLD reactivity to positive, negative, and neutral stimuli in the bilateral amygdala and hippocampus (extracted using AAL MNI ROI library and Marsbar SPM8 ROI toolbox). Bars indicate task‐specific %‐BOLD signal changes (positive, negative, and neutral) for the baseline (green) and ketamine (blue) session (paired t test, n = 23, error bars: ±SEM). [Color figure can be viewed in the online issue, which is available at
.]
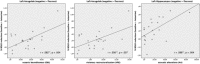
Figure 4
Significant positive correlations between %BOLD signal reductions to negative stimuli and psychedelic alterations of consciousness for the left amygdala and the left hippocampus.
Figure 5
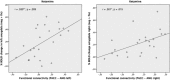
Significant positive correlation between %BOLD signal changes to negative pictures in both amygdalae with functional connectivities to the pgACC during the ketamine condition (AMG left: n = 23, r = 0.530, p = 0.009; AMG right: n = 23, r = 0.501, p = 0.015).
Similar articles
- General and emotion-specific neural effects of ketamine during emotional memory formation.
Becker B, Steffens M, Zhao Z, Kendrick KM, Neumann C, Weber B, Schultz J, Mehta MA, Ettinger U, Hurlemann R. Becker B, et al. Neuroimage. 2017 Apr 15;150:308-317. doi: 10.1016/j.neuroimage.2017.02.049. Epub 2017 Feb 20. Neuroimage. 2017. PMID: 28232170 Clinical Trial. - [Effects of a subanaesthetic dose of ketamine on emotional and behavioral state in healthy subjects].
Micallef J, Tardieu S, Gentile S, Fakra E, Jouve E, Sambuc R, Blin O. Micallef J, et al. Neurophysiol Clin. 2003 Jun;33(3):138-47. doi: 10.1016/s0987-7053(03)00028-5. Neurophysiol Clin. 2003. PMID: 12909392 Clinical Trial. French. - Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood.
Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. Wu M, et al. Hum Brain Mapp. 2016 May;37(5):1684-95. doi: 10.1002/hbm.23129. Epub 2016 Mar 2. Hum Brain Mapp. 2016. PMID: 26931629 Free PMC article. - [Glutaminergic hypothesis of schizophrenia: clinical research studies with ketamine].
Mechri A, Saoud M, Khiari G, d'Amato T, Dalery J, Gaha L. Mechri A, et al. Encephale. 2001 Jan-Feb;27(1):53-9. Encephale. 2001. PMID: 11294039 Review. French. - Review: The use of functional magnetic resonance imaging (fMRI) in clinical trials and experimental research studies for depression.
Kotoula V, Evans JW, Punturieri CE, Zarate CA Jr. Kotoula V, et al. Front Neuroimaging. 2023 Jun 27;2:1110258. doi: 10.3389/fnimg.2023.1110258. eCollection 2023. Front Neuroimaging. 2023. PMID: 37554642 Free PMC article. Review.
Cited by
- Hippocampal volume changes after (R,S)-ketamine administration in patients with major depressive disorder and healthy volunteers.
Evans JW, Graves MC, Nugent AC, Zarate CA Jr. Evans JW, et al. Sci Rep. 2024 Feb 24;14(1):4538. doi: 10.1038/s41598-024-54370-9. Sci Rep. 2024. PMID: 38402253 Free PMC article. - Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration.
Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA Jr. Evans JW, et al. Biol Psychiatry. 2018 Oct 15;84(8):582-590. doi: 10.1016/j.biopsych.2018.01.027. Epub 2018 Feb 15. Biol Psychiatry. 2018. PMID: 29580569 Free PMC article. Clinical Trial. - Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network.
Mueller F, Musso F, London M, de Boer P, Zacharias N, Winterer G. Mueller F, et al. Neuroimage Clin. 2018 Jun 1;19:745-757. doi: 10.1016/j.nicl.2018.05.037. eCollection 2018. Neuroimage Clin. 2018. PMID: 30003027 Free PMC article. Clinical Trial. - Depression in chronic ketamine users: Sex differences and neural bases.
Li CR, Zhang S, Hung CC, Chen CM, Duann JR, Lin CP, Lee TS. Li CR, et al. Psychiatry Res Neuroimaging. 2017 Nov 30;269:1-8. doi: 10.1016/j.pscychresns.2017.09.001. Epub 2017 Sep 5. Psychiatry Res Neuroimaging. 2017. PMID: 28892733 Free PMC article. - The functional connectivity of the middle frontal cortex predicts ketamine's outcome in major depressive disorder.
Zhang F, Wang C, Lan X, Li W, Fu L, Ye Y, Liu H, Wu K, Zhou Y, Ning Y. Zhang F, et al. Front Neurosci. 2022 Sep 15;16:956056. doi: 10.3389/fnins.2022.956056. eCollection 2022. Front Neurosci. 2022. PMID: 36188452 Free PMC article.
References
- Aan Het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010): Safety and efficacy of repeated‐dose intravenous ketamine for treatment‐resistant depression. Biol Psychiatry 67:139–145. - PubMed
- Abel KM, Allin MPG, Kucharska‐Pietura K, David A, Andrew C, Williams S, Brammer MJ, Phillips ML (2003b): Ketamine alters neural processing of facial emotion recognition in healthy men: an fMRI study. Neuroreport 14:387–391. - PubMed
- Aldhafeeri FM, Mackenzie I, Kay T, Alghamdi J, Sluming V (2012): Regional brain responses to pleasant and unpleasant IAPS pictures: different networks. Neurosci Lett 512:94–98. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources