Hypoxia as a therapy for mitochondrial disease - PubMed (original) (raw)
. 2016 Apr 1;352(6281):54-61.
doi: 10.1126/science.aad9642. Epub 2016 Feb 25.
Luca Zazzeron 2, Rahul Goli 1, Kristen Alexa 3, Stephanie Schatzman-Bone 3, Harveen Dhillon 1, Olga Goldberger 1, Jun Peng 1, Ophir Shalem 4, Neville E Sanjana 4, Feng Zhang 4, Wolfram Goessling 5, Warren M Zapol 2, Vamsi K Mootha 6
Affiliations
- PMID: 26917594
- PMCID: PMC4860742
- DOI: 10.1126/science.aad9642
Hypoxia as a therapy for mitochondrial disease
Isha H Jain et al. Science. 2016.
Abstract
Defects in the mitochondrial respiratory chain (RC) underlie a spectrum of human conditions, ranging from devastating inborn errors of metabolism to aging. We performed a genome-wide Cas9-mediated screen to identify factors that are protective during RC inhibition. Our results highlight the hypoxia response, an endogenous program evolved to adapt to limited oxygen availability. Genetic or small-molecule activation of the hypoxia response is protective against mitochondrial toxicity in cultured cells and zebrafish models. Chronic hypoxia leads to a marked improvement in survival, body weight, body temperature, behavior, neuropathology, and disease biomarkers in a genetic mouse model of Leigh syndrome, the most common pediatric manifestation of mitochondrial disease. Further preclinical studies are required to assess whether hypoxic exposure can be developed into a safe and effective treatment for human diseases associated with mitochondrial dysfunction.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Figure 1. Genome-scale Cas9-mediated knockout screen identifies VHL inhibition as protective during states of mitochondrial dysfunction
(A) Mitochondrial disease was modeled with the addition of the complex III inhibitor, antimycin (moderate disease) or antimycin and removal of pyruvate (severe disease). (B) K562 cells were infected in duplicate with the genome-scale Cas9-mediated knockout library, and separated into conditions of untreated, moderate disease or severe disease. Samples were taken at a pre-treatment time point, as well as after three weeks of selection. (C) Growth curves for cumulative differences in growth rates in different experimental conditions for both infection replicates. (D) RIGER output based on enrichment of sgRNAs in severe disease condition relative to pre-treatment conditions. Each row denotes a single gene, with ranks of corresponding sgRNAs in middle column. Ranks for individual sgRNAs are out of ~65,000 total sgRNAs in library. (E) sgRNA enrichment magnitude vs. rank, with most enriched sgRNA shown to the far right. sgRNAs corresponding to VHL in red. (F) Guide abundance in pre-treatment conditions (Infection 1 vs. Infection 2) shown in grey for each sgRNA, representative of experimental noise. Guide abundance in severe disease condition vs. pre-treatment condition in black, with VHL sgRNAs in red.
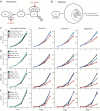
Figure 2. Genetic or small molecule activation of the HIF response is protective against multiple forms of RC inhibition, in multiple cell types
(A) Schematic for HIF degradation during normoxia. (B) Schematic for induction of hypoxia transcriptional program during hypoxia. (C) Growth curves for K562 VHL-knockout cells (cyan, blue) or non-targeting sgRNA cells (black, red) for untreated or disease conditions (mean shown). Disease conditions correspond to inhibition of Complex I (piericidin), Complex III (antimycin) or Complex V (oligomycin). Growth curves for (D) K562 cells, (E) HEK293T cells and (F) HT-29 cells ± FG-4592, in combination with untreated or disease conditions (inhibition of complex I, III and V). All time points were measured in duplicate and all growth curves are representative of 2-3 independent experiments (mean shown). All final cell counts of FG-treated rescue (or VHL-KO rescue in 2A) in presence of RC inhibitor were statistically significant (one-sided t-test p-value < 0.05).
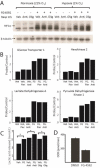
Figure 3. FG-4592 causes normoxic stabilization of HIF1α and rewires energy metabolism
(A) Immunoblot showing HIF1α ± RC inhibition with antimycin or oligomycin, ± FG-4592 under normoxia (21% O2) or hypoxia (1% O2). RC inhibition prevents HIF1α stabilization during hypoxia. FG-4592 administration overcomes this paradox and stabilizes HIF1α even during normoxia. Immunoblot is representative of independent experiments done in duplicate in HT-29 cells. (B) Normalized expression for known HIF targets glucose transporter 1 (GLUT1), hexokinase 2 (HK2), lactate dehydrogenase A (LDHA), and pyruvate dehydrogenase kinase 1 (PDK1) +/− RC inhibition, +/− FG-4592 in HT-29 cells. Data shown as mean of two independent experiments and normalized so vehicle-treated expression (probe/control) is 1. (C) Mean concentration of lactic acid secreted by cells treated with FG-4592 or DMSO ± RC inhibitors as proxy for anaerobic glycolytic flux. Data shown for HEK293T cells (without pyruvate to eliminate contribution from LDH reaction) and is representative of at least two independent experiments (D) Basal oxygen consumption rates for HEK293T cells treated with FG-4592 or DMSO for > 24h, averaged across three independent experiments (Mean ± S.E.). (one-sided t-test p-value < 0.05 for all pairwise comparisons ± FG-4592 in figures 3B-3D).
Figure 4. vhl knockout or FG-4592 treatment activates the HIF response in zebrafish embryos and alleviates death caused by respiratory chain inhibition
(A) 48hpf _vhl_-null zebrafish are less sensitive to RC inhibition than control (WT and Het) fish, n ≥ 75 per treatment, p < 0.001 by Mantel-Cox test. (B) FG-4592 treatment activates expression of HIF-responsive promoter in Tg(phd3::EGFP) embryos. Images are shown for embryos treated with either DMSO or 2.5μM FG-4592 from 96 to 102hpf. Embryos were assayed for GFP expression at 0 hours post treatment (hptx) and 6hptx. DMSO treatment fails to activate GFP expression beyond autofluorescence in Tg(phd3::EGFP) transgenic embryos, while FG-4592 robustly initiates GFP expression by 6hptx. (C) Known Hif targets, glut1 and ldha1 are overexpressed in 96hpf zebrafish embryos treated with FG-4592 for 6h. (D) Exposure to FG-4592 rescues antimycin-induced zebrafish embryonic death. Respiratory chain inhibition by 2.5nM antimycin in 4dpf (days post fertilization) embryos results in significant death within the first 24 hours of treatment. Co-exposure of antimycin with FG-4592 (2.5μM) doubles embryo survival, while FG-4592 alone has no impact. n=75 per treatment, p < 0.0001 by Mantel-Cox test.
Figure 5. Chronic hypoxia extends lifespan and alleviates disease in a mouse model of Leigh syndrome, whereas chronic hyperoxia exacerbates disease
(A) Ndufs4 KO mice of both genders were chronically exposed to hypoxia (11% O2), normoxia (21% O2) or hyperoxia (55% O2), at 30d of age and survival was recorded (n = 12, n = 12, n = 9 mice respectively). Cyan bars represent current age of hypoxic KO mice. (B) Body weights were measured in WT and KO mice exposed to normoxia or hypoxia, three times a week upon enrollment in the study. Weights are shown as mean ± S.E. (C) Representative images of 50d-old KO mice exposed to normoxia or hypoxia. (D) Body temperature was measured in KO mice exposed to normoxia or hypoxia at age ~30d, 40d and 50d. Temperatures are shown as mean ± S.E. (n ≥ 7 for all groups) (E) Latency to fall on an accelerating rod was measured as median values of triplicate trials per mouse for WT and KO mice, exposed to normoxia or hypoxia at different ages (n ≥ 7 for all groups). (F) Representative 1h locomotor activity traces of sick, normoxia-treated KO mice and age-matched hypoxia-treated KO mice, as well as controls. All data shown as normoxia KO (maroon), hypoxia KO (blue), normoxia WT (black) and hypoxia WT (grey). *denotes t-test p-value < 0.05.
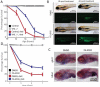
Figure 6. Hypoxia exposure of Ndufs4 KO mice alleviates metabolic disease markers, as well as neuropathology, without rescuing Complex I activity
(A) Hematocrit values for WT and KO mice treated with normoxia or hypoxia for ~3 weeks (n = 3-4 per group, test p-value < 0.05 for normoxia vs. hypoxia for both WT and KO). (B) Complex I Activity is significantly reduced in KO mice relative to WT mice, in both normoxic and hypoxic conditions (n = 3-4 per group, t-test p-value < 0.01). (C) Representative images for immunostaining against the inflammatory marker, Iba-1, in the olfactory bulb and cerebellum of Ndufs4 KO mice treated with hypoxia or normoxia and WT mice exposed to normoxia breathing. The number of Iba-1 positive cells per 10 random fields of view shown for each treatment group (Mean ± S.E., t-test p-value < 0.01 for normoxic vs. hypoxic KO, n = 3-4 per group). Scale bar is 200 microns for OB and 50 microns for cerebellum. (D) Plasma α-HB levels in WT and KO mice, exposed to hypoxia or normoxia (n = 4-8 per group). Median shown as horizontal bar. (E) Plasma lactate in WT and KO mice, exposed to hypoxia or normoxia (n = 4-8 per group). Median shown as horizontal bar.
Comment in
- MITOCHONDRIA. Mitochondrial disease therapy from thin air?
Shoubridge EA. Shoubridge EA. Science. 2016 Apr 1;352(6281):31-2. doi: 10.1126/science.aaf5248. Epub 2016 Mar 31. Science. 2016. PMID: 27034357 No abstract available. - Applying the Airbrakes: Treating Mitochondrial Disease with Hypoxia.
Russell OM, Lightowlers RN, Turnbull DM. Russell OM, et al. Mol Cell. 2016 Apr 7;62(1):5-6. doi: 10.1016/j.molcel.2016.03.027. Mol Cell. 2016. PMID: 27058784
Similar articles
- Applying the Airbrakes: Treating Mitochondrial Disease with Hypoxia.
Russell OM, Lightowlers RN, Turnbull DM. Russell OM, et al. Mol Cell. 2016 Apr 7;62(1):5-6. doi: 10.1016/j.molcel.2016.03.027. Mol Cell. 2016. PMID: 27058784 - MITOCHONDRIA. Mitochondrial disease therapy from thin air?
Shoubridge EA. Shoubridge EA. Science. 2016 Apr 1;352(6281):31-2. doi: 10.1126/science.aaf5248. Epub 2016 Mar 31. Science. 2016. PMID: 27034357 No abstract available. - Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome.
Ferrari M, Jain IH, Goldberger O, Rezoagli E, Thoonen R, Cheng KH, Sosnovik DE, Scherrer-Crosbie M, Mootha VK, Zapol WM. Ferrari M, et al. Proc Natl Acad Sci U S A. 2017 May 23;114(21):E4241-E4250. doi: 10.1073/pnas.1621511114. Epub 2017 May 8. Proc Natl Acad Sci U S A. 2017. PMID: 28483998 Free PMC article. - Hypoxia-inducible factor 1 and cancer pathogenesis.
Semenza GL. Semenza GL. IUBMB Life. 2008 Sep;60(9):591-7. doi: 10.1002/iub.93. IUBMB Life. 2008. PMID: 18506846 Review. - Zebrafish as a model for von Hippel Lindau and hypoxia-inducible factor signaling.
Kim HR, Greenald D, Vettori A, Markham E, Santhakumar K, Argenton F, van Eeden F. Kim HR, et al. Methods Cell Biol. 2017;138:497-523. doi: 10.1016/bs.mcb.2016.07.001. Epub 2016 Sep 6. Methods Cell Biol. 2017. PMID: 28129856 Review.
Cited by
- Maternally inherited mitochondrial respiratory disorders: from pathogenetic principles to therapeutic implications.
Uittenbogaard M, Chiaramello A. Uittenbogaard M, et al. Mol Genet Metab. 2020 Sep-Oct;131(1-2):38-52. doi: 10.1016/j.ymgme.2020.06.011. Epub 2020 Jun 27. Mol Genet Metab. 2020. PMID: 32624334 Free PMC article. Review. - Mechanisms of Mitochondrial ROS Production in Assisted Reproduction: The Known, the Unknown, and the Intriguing.
Cobley JN. Cobley JN. Antioxidants (Basel). 2020 Sep 29;9(10):933. doi: 10.3390/antiox9100933. Antioxidants (Basel). 2020. PMID: 33003362 Free PMC article. Review. - Hypoxia Promotes Mitochondrial Complex I Abundance via HIF-1α in Complex III and Complex IV Eficient Cells.
Saldana-Caboverde A, Nissanka N, Garcia S, Lombès A, Diaz F. Saldana-Caboverde A, et al. Cells. 2020 Sep 29;9(10):2197. doi: 10.3390/cells9102197. Cells. 2020. PMID: 33003371 Free PMC article. - Interactions of mitochondrial and skeletal muscle biology in mitochondrial myopathy.
Di Leo V, Bernardino Gomes TM, Vincent AE. Di Leo V, et al. Biochem J. 2023 Nov 15;480(21):1767-1789. doi: 10.1042/BCJ20220233. Biochem J. 2023. PMID: 37965929 Free PMC article. Review. - Bezafibrate Rescues Mitochondrial Encephalopathy in Mice via Induction of Daily Torpor and Hypometabolic State.
Lyu J, Zhao Y, Zhang N, Xu X, Zheng R, Yu W, Xin W, Yan C, Ji K. Lyu J, et al. Neurotherapeutics. 2022 Apr;19(3):994-1006. doi: 10.1007/s13311-022-01216-9. Epub 2022 Mar 25. Neurotherapeutics. 2022. PMID: 35334081 Free PMC article.
References
- Rich P. Chemiosmotic coupling: the cost of living. Nature. 2003;421:583. - PubMed
- Vafai SB, Mootha VK. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–383. - PubMed
- Koopman WJ, Willems PH, Smeitink JA. Monogenic mitochondrial disorders. N. Engl. J. Med. 2012;366:1132–1141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK090311/DK/NIDDK NIH HHS/United States
- R24 OD017870/OD/NIH HHS/United States
- R01 MH110049/MH/NIMH NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- K99 HG008171/HG/NHGRI NIH HHS/United States
- 5DP1-MH100706/DP/NCCDPHP CDC HHS/United States
- K99-HG008171/HG/NHGRI NIH HHS/United States
- 1R01-MH110049/MH/NIMH NIH HHS/United States
- DP1 MH100706/MH/NIMH NIH HHS/United States
- Howard Hughes Medical Institute/United States
- R01 DK097768/DK/NIDDK NIH HHS/United States
- 5R01DK097768-03/DK/NIDDK NIH HHS/United States
- R01 DK090311/DK/NIDDK NIH HHS/United States
- R24OD017870/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases