CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity - PubMed (original) (raw)
CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity
Melanie Straub et al. Oncotarget. 2016.
Abstract
Immunomodulatory therapies, targeting the immune checkpoint receptor-ligand complex PD-1/PD-L1 have shown promising results in early phase clinical trials in solid malignancies, including carcinomas of the head and neck. In this context, PD-L1 protein expression has been proposed as a potentially valuable predictive marker. In the present study, expression of PD-L1 and PD-1 was evaluated by immunohistochemistry in 80 patients with predominantly HPV-negative oral squamous cell carcinomas and associated nodal metastasis. In addition, CD274/PD-L1 gene copy number status was assessed by fluorescence in situ hybridization analysis. PD-L1 expression was detected in 36/80 (45%) cases and concordance of PD-L1 expression in primary tumor and corresponding nodal metastasis was present in only 20/28 (72%) cases. PD-1 expression was found in tumor-infiltrating lymphocytes (TILs) but not in tumor cells. CD274/PD-L1 gene amplification was detected in 19% of cases, with high level PD-L1 amplification present in 12/80 (15%), and low level amplification in 3/80 (4%). Interestingly, CD274/PD-L1 gene amplification was associated with positive PD-L1 immunostaining in only 73% of cases. PD-L1 copy number status was concordant in primary tumor and associated metastases. Clinically, PD-L1 tumor immunopositivity was associated with a higher risk for nodal metastasis at diagnosis, overall tumor related death und recurrence. Based on our findings we propose to include PD-L1 copy number status in addition to protein status in screening programs for future clinical trials with immunotherapeutic strategies targeting the PD-1/PD-L1 axis.
Keywords: PD-1; PD-L1; Pathology Section; fluorescence in situ hybridization; gene amplification; oral squamous cell carcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Figures
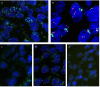
Figure 1. Squamous cell carcinoma of the head and neck, showing membraneous PD-L1 expression in tumor cells with
a. strong staining (3+), b. intermediate staining (2+) and c. weak staining (1+) intensity. Fibroblasts within tumor stroma are negative. d) PD-1 staining in squamous cell carcinoma with intermingled tumor-infiltrating lymphocytes (TILs). PD-1 staining is only seen in TILs, while carcinoma cells are negative. (a.-c. PD-L1 immunohistochemistry, d. PD-1 immunohistochemistry).
Figure 2. Concordance and discordance of PD-L1 expression in primary tumor and matched lymph node metastases
Figure 3. Fluorescence _in situ_-hybridization (FISH) for PD-L1
PD-L1 gene is labelled in green, centromer 9 in red. FISH analysis showing a., b. high level amplification, with PD-L1/CEP9 ratio ≥ 4, indicated by clusters of green fluorochrome labeling PD-L1 c. low level amplification, PD-L1/CEP9 ratio ≥ 2.0 - < 4; d. Polysomy of PD-L1 gene locus; e. PD-L1 disomy. (a.-d. SPEC _CD274, PDCD1LG2/CEN_9 Dual Color Probe).
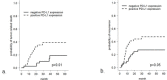
Figure 4. Overall tumor-related survival and recurrence-free survival in patients with SCC of the oral cavity with respect to PD-L1 immunohistochemical status
Overall tumor-related survival a. and recurrence-free survival b. is worse in SCC patients with tumoral PD-L1 immunopositivity (p = 0.01 and 0.05, respectively).
Similar articles
- PD-L1 expression predicts longer disease free survival in high risk head and neck cutaneous squamous cell carcinoma.
Roper E, Lum T, Palme CE, Ashford B, Ch'ng S, Ranson M, Boyer M, Clark J, Gupta R. Roper E, et al. Pathology. 2017 Aug;49(5):499-505. doi: 10.1016/j.pathol.2017.04.004. Epub 2017 Jun 27. Pathology. 2017. PMID: 28666643 - Programmed cell death-ligand 1 expression in oral squamous cell carcinoma is associated with an inflammatory phenotype.
Satgunaseelan L, Gupta R, Madore J, Chia N, Lum T, Palme CE, Boyer M, Scolyer RA, Clark JR. Satgunaseelan L, et al. Pathology. 2016 Oct;48(6):574-80. doi: 10.1016/j.pathol.2016.07.003. Epub 2016 Aug 30. Pathology. 2016. PMID: 27590194 - PD-1 Expression in Head and Neck Squamous Cell Carcinomas Derives Primarily from Functionally Anergic CD4+ TILs in the Presence of PD-L1+ TAMs.
Mattox AK, Lee J, Westra WH, Pierce RH, Ghossein R, Faquin WC, Diefenbach TJ, Morris LG, Lin DT, Wirth LJ, Lefranc-Torres A, Ishida E, Chakravarty PD, Johnson L, Zeng YC, Chen H, Poznansky MC, Iyengar NM, Pai SI. Mattox AK, et al. Cancer Res. 2017 Nov 15;77(22):6365-6374. doi: 10.1158/0008-5472.CAN-16-3453. Epub 2017 Sep 25. Cancer Res. 2017. PMID: 28947422 Free PMC article. - Turning the tide: Clinical utility of PD-L1 expression in squamous cell carcinoma of the head and neck.
De Meulenaere A, Vermassen T, Aspeslagh S, Huvenne W, Van Dorpe J, Ferdinande L, Rottey S. De Meulenaere A, et al. Oral Oncol. 2017 Jul;70:34-42. doi: 10.1016/j.oraloncology.2017.05.002. Epub 2017 May 19. Oral Oncol. 2017. PMID: 28622889 Review. - An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives.
Lenouvel D, González-Moles MÁ, Talbaoui A, Ramos-García P, González-Ruiz L, Ruiz-Ávila I, Gil-Montoya JA. Lenouvel D, et al. Oral Dis. 2020 Apr;26(3):511-526. doi: 10.1111/odi.13088. Epub 2019 Apr 10. Oral Dis. 2020. PMID: 30866171 Review.
Cited by
- Prognostic value of tumor-infiltrating immune cells and immune checkpoints in elderly head-and-neck squamous cell carcinoma patients undergoing definitive (chemo)radiotherapy.
Rühle A, Todorovic J, Spohn SSK, Gkika E, Becker C, Knopf A, Zamboglou C, Sprave T, Werner M, Grosu AL, Kayser G, Nicolay NH. Rühle A, et al. Radiat Oncol. 2022 Nov 14;17(1):181. doi: 10.1186/s13014-022-02153-9. Radiat Oncol. 2022. PMID: 36376922 Free PMC article. - Comprehensive characterization of B7 family members in NSCLC and identification of its regulatory network.
Xiao M, Pang C, Xiang S, Zhao Y, Wu X, Li M, Du F, Chen Y, Wang F, Wen Q, Xiao Z, Yang Z, Shen J. Xiao M, et al. Sci Rep. 2023 Mar 15;13(1):4311. doi: 10.1038/s41598-022-26776-w. Sci Rep. 2023. PMID: 36922519 Free PMC article. - [Molecular predictors in immune oncology].
Weichert W. Weichert W. Pathologe. 2018 Nov;39(6):546-555. doi: 10.1007/s00292-018-0508-9. Pathologe. 2018. PMID: 30215118 Review. German. - Prognosis Value of Immunoregulatory Molecules in Oral Cancer Microenvironment: An Immunohistochemical Study.
Peña-Cardelles JF, Pozo-Kreilinger JJ, Roncador G, Esteban-Hernández J, Moro-Rodríguez JE, Sastre-Perona A, Castelo-Fernández B, Cebrián-Carretero JL. Peña-Cardelles JF, et al. Biomedicines. 2022 Mar 19;10(3):710. doi: 10.3390/biomedicines10030710. Biomedicines. 2022. PMID: 35327512 Free PMC article. - Prognostic value of immune checkpoint molecules in head and neck cancer: a meta-analysis.
Jia YQ, Yang B, Wen LL, Mu WX, Wang Z, Cheng B. Jia YQ, et al. Aging (Albany NY). 2019 Jan 22;11(2):501-522. doi: 10.18632/aging.101756. Aging (Albany NY). 2019. PMID: 30668545 Free PMC article.
References
- Pfister DG. Head and Neck Cancers, Version 2. 2013 Featured Updates to the NCCN Guidelines (vol 11, pg 917, 2013) J Natl Compr Canc Ne. 2013;11:1458–1458. - PubMed
- Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, Yip P, Yu B, O'Toole SA, McCaughan BC, Yearley JH, Horvath LG, Kao S, Boyer M, Scolyer RA. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials