A comparative view of regenerative neurogenesis in vertebrates - PubMed (original) (raw)
Review
A comparative view of regenerative neurogenesis in vertebrates
Alessandro Alunni et al. Development. 2016.
Abstract
In all vertebrate species studied thus far, the adult central nervous system harbors neural stem cells that sustain constitutive neurogenesis, as well as latent neural progenitors that can be awakened in lesional contexts. In spite of this common theme, many species differ dramatically in their ability to recruit constitutive progenitors, to awaken latent progenitors, or to enhance or bias neural progenitor fate to achieve successful neuronal repair. This Review summarizes the striking similarities in the essential molecular and cellular properties of adult neural stem cells between different vertebrate species, both under physiological and reparative conditions. It also emphasizes the differences in the reparative process across evolution and how the study of non-mammalian models can provide insights into both basic neural stem cell properties and stimulatory cues shared between vertebrates, and subsequent neurogenic events, which are abortive under reparative conditions in mammals.
Keywords: Lesion; Neural stem cells; Neurogenesis; Repair; Zebrafish.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests
The authors declare no competing or financial interests.
Figures
Fig. 1.
Phylogenetic tree of animal taxons used as models for neuronal regeneration. The location of adult neurogenic niches, which harbor constitutively active neuronal progenitors (red), and the presence of latent neural progenitors (blue) are indicated on schematic sagittal sections of the brain (left). Constitutive neurogenesis generates neurons in the adult brain under homeostatic conditions, whereas latent progenitors are activated in response to lesions to produce neurons and/or glial cells. The table summarizes the presence of (+), the demonstrated absence of (−), or the lack of experimental data on (?) constitutive neuronal progenitors, latent neural progenitors and reparative neurogenesis in the different central nervous system regions discussed throughout this Review. F, forebrain; M, midbrain; Sc, spinal cord; R, retina.
Fig. 2.
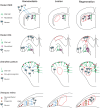
Neuronal repair from niche progenitors. In the rodent subependymal zone (SEZ), glial cells give rise to neuroblasts that migrate along the rostral migratory stream into the olfactory bulb (OB) to generate unique types of interneurons (left panel, blue arrows). Stroke injury (red outline) results in localized cell death in the striatum and the proliferation of endogenous neural progenitor cells that migrate from the SEZ to the striatum to elicit regeneration (right panel, red arrows). This migration occurs at the expense of normal neuroblast migration from the SEZ to OB. In the rodent dentate gyrus (DG), radial glial cells produce transit-amplifying progenitors, called neuroblasts, which generate neurons (left panel). These newborn neurons migrate into the granule cell layer (blue arrows). Ischemia (center panel, red outline) induces the degeneration of pyramidal neurons. Following the ischemia the endogenous progenitors proliferate and subsequently migrate to regenerate new neurons (right panel, red arrows). The ventricular zone of the adult zebrafish pallium consists predominantly of radial glial cells, which act as self-renewing and multipotent progenitors (left panel). In addition, non-glial cycling neuroblasts are intermingled along the ventricle. Together, radial glia and neuroblasts generate pallial neurons (left panel, blue arrows). Reactive neurogenesis has been induced in the zebrafish adult pallium mostly by mechanical injury using stab lesion causing a circumscribed injury in the parenchyma of the telencephalon without injuring ventricular lining (center panel, red outline). In response, neuroblasts and radial glia increase their proliferation to produce neurons to compensate for the neuronal loss (right panel, red and green arrows, respectively). In the amphibian retina, the ciliary marginal zone (CMZ) continuously generates all neuronal subtypes (left panel, blue arrows). Upon extensive lesion in X. tropicalis (center panel, red outline), the CMZ is activated to elicit regeneration (right panel, red arrows). In the rodent and zebrafish schematics, only the left hemisphere is depicted.
Fig. 3.
Neuronal repair from latent progenitors. In the zebrafish retina, new retinal neurons are generated sequentially from retinal stem cells located in the ciliary marginal zone (CMZ). Under homeostatic conditions, the Müller glia cells (MGs) generate only rod precursors, which give rise to rod photoreceptors (left panel, green and blue arrows). Following lesion (center panel, red outline), MGs re-enter the cell cycle and divide once asymmetrically to generate neurogenic clusters that go on to produce all missing neurons (right panel, green arrows). In the rodent striatum, the astrocytes are not neurogenic (left panel). After a stroke (center panel, red outline), some striatal astrocytes generate neuroblasts that give rise to a limited number of new neurons (right panel, green arrows). In the newt midbrain under homeostatic conditions, ependymoglial cells are quiescent (left panel). A selective neurotoxin administered intraventricularly selectively eliminates midbrain dopaminergic neurons (center panel, red outline), inducing the proliferation of the ependymoglial cells, which generate new dopaminergic neurons (right panel, green arrows). Ependymoglial cells in zebrafish spinal cord are self-renewing and give rise to oligodendrocytes (left panel, red arrows). After a lesion (center panel, red outline), these cells divide, migrate and produce new motor neurons (right panel, green arrows).
Similar articles
- Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment.
Ceci M, Mariano V, Romano N. Ceci M, et al. Rev Neurosci. 2018 Dec 19;30(1):45-66. doi: 10.1515/revneuro-2018-0020. Rev Neurosci. 2018. PMID: 30067512 Review. - Radial glia and neural progenitors in the adult zebrafish central nervous system.
Than-Trong E, Bally-Cuif L. Than-Trong E, et al. Glia. 2015 Aug;63(8):1406-28. doi: 10.1002/glia.22856. Epub 2015 May 14. Glia. 2015. PMID: 25976648 Review. - Differential expression of id genes and their potential regulator znf238 in zebrafish adult neural progenitor cells and neurons suggests distinct functions in adult neurogenesis.
Diotel N, Beil T, Strähle U, Rastegar S. Diotel N, et al. Gene Expr Patterns. 2015 Sep-Nov;19(1-2):1-13. doi: 10.1016/j.gep.2015.05.004. Epub 2015 Jun 21. Gene Expr Patterns. 2015. PMID: 26107416 - Characterization of Proliferating Neural Progenitors after Spinal Cord Injury in Adult Zebrafish.
Hui SP, Nag TC, Ghosh S. Hui SP, et al. PLoS One. 2015 Dec 2;10(12):e0143595. doi: 10.1371/journal.pone.0143595. eCollection 2015. PLoS One. 2015. PMID: 26630262 Free PMC article. - Role of the immune response in initiating central nervous system regeneration in vertebrates: learning from the fish.
Bosak V, Murata K, Bludau O, Brand M. Bosak V, et al. Int J Dev Biol. 2018;62(6-7-8):403-417. doi: 10.1387/ijdb.180033vb. Int J Dev Biol. 2018. PMID: 29938753 Review.
Cited by
- Neuron-Radial Glial Cell Communication via BMP/Id1 Signaling Is Key to Long-Term Maintenance of the Regenerative Capacity of the Adult Zebrafish Telencephalon.
Zhang G, Lübke L, Chen F, Beil T, Takamiya M, Diotel N, Strähle U, Rastegar S. Zhang G, et al. Cells. 2021 Oct 19;10(10):2794. doi: 10.3390/cells10102794. Cells. 2021. PMID: 34685774 Free PMC article. - Mechanisms of Pathology-Induced Neural Stem Cell Plasticity and Neural Regeneration in Adult Zebrafish Brain.
Kizil C. Kizil C. Curr Pathobiol Rep. 2018;6(1):71-77. doi: 10.1007/s40139-018-0158-x. Epub 2018 Jan 16. Curr Pathobiol Rep. 2018. PMID: 29938129 Free PMC article. Review. - Repair after brainstem ischemia involves neurogenesis and the rubrospinal system.
Rüber T, Schlaug G. Rüber T, et al. Ann Neurol. 2018 Jun;83(6):1069-1071. doi: 10.1002/ana.25265. Ann Neurol. 2018. PMID: 29908075 Free PMC article. No abstract available. - Regulation of Stem Cell Properties of Müller Glia by JAK/STAT and MAPK Signaling in the Mammalian Retina.
Beach KM, Wang J, Otteson DC. Beach KM, et al. Stem Cells Int. 2017;2017:1610691. doi: 10.1155/2017/1610691. Epub 2017 Jan 17. Stem Cells Int. 2017. PMID: 28194183 Free PMC article. Review. - Post-Traumatic Expressions of Aromatase B, Glutamine Synthetase, and Cystathionine-Beta-Synthase in the Cerebellum of Juvenile Chum Salmon, Oncorhynchus keta.
Pushchina EV, Bykova ME, Varaksin AA. Pushchina EV, et al. Int J Mol Sci. 2024 Mar 14;25(6):3299. doi: 10.3390/ijms25063299. Int J Mol Sci. 2024. PMID: 38542274 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources