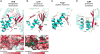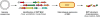New Insights into the Biosynthetic Logic of Ribosomally Synthesized and Post-translationally Modified Peptide Natural Products - PubMed (original) (raw)
Review
New Insights into the Biosynthetic Logic of Ribosomally Synthesized and Post-translationally Modified Peptide Natural Products
Manuel A Ortega et al. Cell Chem Biol. 2016.
Abstract
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are a large group of structurally diverse natural products. Their biological activities and unique biosynthetic pathways have sparked a growing interest in RiPPs. Furthermore, the relatively low genetic complexity associated with RiPP biosynthesis makes them excellent candidates for synthetic biology applications. This Review highlights recent developments in the understanding of the biosynthesis of several bacterial RiPP family members, the use of the RiPP biosynthetic machinery for generating novel macrocyclic peptides, and the implementation of tools designed to guide the discovery and characterization of novel RiPPs.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
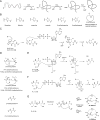
Figure 1
A window into RiPP biosynthesis. (A) Schematic RiPP biosynthetic pathway. Representative RiPP biosynthetic gene cluster where each gene encodes a biosynthetic enzyme color-coded by the modification it catalyzes. Dotted circles represent amino acids within the leader peptide. Full circles represent amino acids within the core region. (B) Chemical structures of select RiPP subfamily members. Structural features defining a particular RiPP subfamily are shown in blue. Additional PTMs are shown in red.
Figure 2
Overview of PTMs in lasso peptides, cyanobactins, and lanthipeptides. (A) Proposed biosynthetic scheme during the maturation of the lasso peptide microcin J25. Leader peptide is shown as dotted circles; core peptide is shown as a line. (B) PTMs often present in cyanobactins. (C) Proposed mechanism for the formation of azolines in cyanobactins catalyzed by YcaO-like cyclodehydratases. (D) Common PTMs found in lanthipeptides. (E–F) Proposed dehydration mechanism employed by (E) class I lanthipeptide dehydratases and (F) class II–IV lanthionine synthetases. (G) Proposed cyclization mechanism in lanthipeptides. Xn, connecting peptide.
Figure 3
Overview of PTMs in thiopeptides, sactipeptides, and streptide. (A) Putative macrocyclization mechanism involved in the generation of the characteristic central six-membered nitrogen-containing heterocycle in thiopeptides. To date no direct evidence has been provided favoring either a concerted or stepwise mechanism. (B) Proposed steps involved in the generation of MIA during nosiheptide biosynthesis catalyzed by NosL. (C) Trp methylation catalyzed by TsrM during thiostrepton biosynthesis. (D–E) Overview of SAM dependent transformations during (D) sactipeptide and (E) streptide biosynthesis. Xn, connecting peptide.
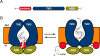
Figure 4
Leader peptide removal by RiPP PCATs. (A) General domain architecture of PCAT enzymes. (B) Proposed model of leader peptide removal with concomitant transport catalyzed by PCATs. (left) Cartoon representing the complex in its ATP-free (left) and ATP-bound (right) forms. Figure adapted from (Lin et al., 2015). TMD, transmembrane domain; NBD, nucleotide binding domain.
Figure 5
Recognition of the leader peptide by the RiPP biosynthetic machinery. (A–B) Cartoon (top) and surface (bottom) representation of the wHTH motif in (A) NisB in complex with NisA (PDB ID 4WD9), and (B) LynD in complex with PatE’ (PDB ID 4V1T). PatE’ and NisA leader peptide are shown in red (top) or green (bottom). (C–D) Cartoon representation of (C) PqqD (PDB ID 3G2B), and of (D) the putative leader peptide-binding site in CylM (PDB ID 5DZT). In all structures α helices are shown in cyan and β sheets are shown in magenta.
Figure 6
General strategy for RiPP genome mining and structural characterization. BGC, biosynthetic gene cluster.
Similar articles
- A prevalent peptide-binding domain guides ribosomal natural product biosynthesis.
Burkhart BJ, Hudson GA, Dunbar KL, Mitchell DA. Burkhart BJ, et al. Nat Chem Biol. 2015 Aug;11(8):564-70. doi: 10.1038/nchembio.1856. Epub 2015 Jul 13. Nat Chem Biol. 2015. PMID: 26167873 Free PMC article. - Recent Advances in the Discovery and Biosynthetic Study of Eukaryotic RiPP Natural Products.
Luo S, Dong SH. Luo S, et al. Molecules. 2019 Apr 18;24(8):1541. doi: 10.3390/molecules24081541. Molecules. 2019. PMID: 31003555 Free PMC article. Review. - Ribosomally synthesized and post-translationally modified peptide natural products: new insights into the role of leader and core peptides during biosynthesis.
Yang X, van der Donk WA. Yang X, et al. Chemistry. 2013 Jun 10;19(24):7662-77. doi: 10.1002/chem.201300401. Epub 2013 May 10. Chemistry. 2013. PMID: 23666908 Free PMC article. - Ribosomally synthesized and post-translationally modified peptide natural product discovery in the genomic era.
Hetrick KJ, van der Donk WA. Hetrick KJ, et al. Curr Opin Chem Biol. 2017 Jun;38:36-44. doi: 10.1016/j.cbpa.2017.02.005. Epub 2017 Mar 2. Curr Opin Chem Biol. 2017. PMID: 28260651 Free PMC article. Review. - Discovery of novel fungal RiPP biosynthetic pathways and their application for the development of peptide therapeutics.
Vogt E, Künzler M. Vogt E, et al. Appl Microbiol Biotechnol. 2019 Jul;103(14):5567-5581. doi: 10.1007/s00253-019-09893-x. Epub 2019 May 31. Appl Microbiol Biotechnol. 2019. PMID: 31147756 Review.
Cited by
- A structurally conserved helix enables leader-independent tyramine splicing of proteins.
Richter D, Courvoisier-Clément A, Vagstad AL, Magyari S, Piel J. Richter D, et al. Chem Sci. 2024 Sep 18;15(40):16645-50. doi: 10.1039/d4sc03867c. Online ahead of print. Chem Sci. 2024. PMID: 39309086 Free PMC article. - Trapping a cross-linked lysine-tryptophan radical in the catalytic cycle of the radical SAM enzyme SuiB.
Balo AR, Caruso A, Tao L, Tantillo DJ, Seyedsayamdost MR, Britt RD. Balo AR, et al. Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2101571118. doi: 10.1073/pnas.2101571118. Proc Natl Acad Sci U S A. 2021. PMID: 34001621 Free PMC article. - Genome mining strategies for ribosomally synthesised and post-translationally modified peptides.
Russell AH, Truman AW. Russell AH, et al. Comput Struct Biotechnol J. 2020 Jun 25;18:1838-1851. doi: 10.1016/j.csbj.2020.06.032. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32728407 Free PMC article. Review. - Cell-Free Systems: Ideal Platforms for Accelerating the Discovery and Production of Peptide-Based Antibiotics.
Park H, Jin H, Kim D, Lee J. Park H, et al. Int J Mol Sci. 2024 Aug 22;25(16):9109. doi: 10.3390/ijms25169109. Int J Mol Sci. 2024. PMID: 39201795 Free PMC article. Review. - Understanding the Mechanism of Action of NAI-112, a Lanthipeptide with Potent Antinociceptive Activity.
Tocchetti A, Iorio M, Hamid Z, Armirotti A, Reggiani A, Donadio S. Tocchetti A, et al. Molecules. 2021 Nov 9;26(22):6764. doi: 10.3390/molecules26226764. Molecules. 2021. PMID: 34833857 Free PMC article.
References
- Abts A, Montalban-Lopez M, Kuipers OP, Smits SH, Schmitt L. NisC binds the FxLx motif of the nisin leader peptide. Biochemistry. 2013;52:5387–95. - PubMed
- Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30:108–60. - PMC - PubMed
- Beck-Sickinger AG, Jung G. Synthesis and conformational analysis of lantibiotic leader-, pro-, and pre-peptides. In: JUNG G, SAHL H-G, editors. Nisin and Novel Lantibiotics. Leiden: ESCOM; 1991.
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM058822/GM/NIGMS NIH HHS/United States
- R37 GM058822/GM/NIGMS NIH HHS/United States
- T32 GM070421/GM/NIGMS NIH HHS/United States
- R01GM0058822/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources