Autophagy interaction with herpes simplex virus type-1 infection - PubMed (original) (raw)
Review
Autophagy interaction with herpes simplex virus type-1 infection
Douglas O'Connell et al. Autophagy. 2016.
Abstract
More than 50% of the U.S. population is infected with herpes simplex virus type-I (HSV-1) and global infectious estimates are nearly 90%. HSV-1 is normally seen as a harmless virus but debilitating diseases can arise, including encephalitis and ocular diseases. HSV-1 is unique in that it can undermine host defenses and establish lifelong infection in neurons. Viral reactivation from latency may allow HSV-1 to lay siege to the brain (Herpes encephalitis). Recent advances maintain that HSV-1 proteins act to suppress and/or control the lysosome-dependent degradation pathway of macroautophagy (hereafter autophagy) and consequently, in neurons, may be coupled with the advancement of HSV-1-associated pathogenesis. Furthermore, increasing evidence suggests that HSV-1 infection may constitute a gradual risk factor for neurodegenerative disorders. The relationship between HSV-1 infection and autophagy manipulation combined with neuropathogenesis may be intimately intertwined demanding further investigation.
Keywords: ICP34.5; autophagy; herpes simplex virus; immune evasion; neurodegeneration.
Figures
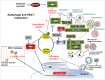
Figure 1.
The autophagy pathway and its interaction with HSV-1. Upon HSV-1 infection, autophagy is stimulated through the activation of an IFN-inducible EIF2AK2/PKR-EIF2S1/eIF2α signaling cascade, which shuts off host protein synthesis and concomitantly turns on autophagy by hitherto unclear mechanisms (indicated by the question mark). Autophagy induction sequesters cytoplasmic contents, forming autophagosomes characterized by the LC3-I → LC3-II conversion and ATG12–ATG5-ATG16L1 supercomplex association. As lysosomes and/or endosomes fuse, many factors contribute to the formation of the autolysosome, enabling degradation of contents by hydrolytic enzymes. Digested materials can be recycled back into the cytosol for use in energy production, protein manufacturing or be delivered to antigen presentation pathways in response to infection. As such, autophagy is shown to be able to directly capture the neuroattenuated ICP34.5-mutant HSV-1 virions or viral components, delivering them for lysosomal degradation and/or for the antigen presentation of viral peptides to the MHC-I/-II pathway for adaptive immune activation. To counteract the antiviral role of EIF2AK2 and cellular autophagy, viral protein Us11 prevents EIF2AK2-mediated EIF2S1 phosphorylation. Interestingly, ICP34.5 acts to reverse phosphorylated EIF2S1 by recruiting of host phosphatase PPP1CA/PP1α. In addition, ICP34.5 restricts autophagic initiation and maturation by targeting BECN1, preventing BECN1 autophagy complex formation. ICP34.5 also engages TBK1 to inhibit TBK1-mediated antiviral signaling, and may also prevent autophagic cargo recruitment through TBK1-mediated SQSTM1 phosphorylation. Although the response of nuclear envelope-derived autophagosomes (NEDA) can be triggered by ICP34.5-associated active protein translation or independently by expression of abundant viral late proteins (e.g., gH [glycoprotein H]), ICP34.5 can restrict the NEDA maturation that engages in viral antigen presentation. The interplay between the herpes pathogen and its host cell reflects a constant battle for control. RUBCN, RUN domain and cysteine-rich domain containing, Beclin 1-interacting protein; VPS, vacuolar protein sorting.
Similar articles
- Herpes Simplex Virus and Interferon Signaling Induce Novel Autophagic Clusters in Sensory Neurons.
Katzenell S, Leib DA. Katzenell S, et al. J Virol. 2016 Apr 14;90(9):4706-4719. doi: 10.1128/JVI.02908-15. Print 2016 May. J Virol. 2016. PMID: 26912623 Free PMC article. - Autophagy stimulation abrogates herpes simplex virus-1 infection.
Yakoub AM, Shukla D. Yakoub AM, et al. Sci Rep. 2015 Apr 9;5:9730. doi: 10.1038/srep09730. Sci Rep. 2015. PMID: 25856282 Free PMC article. - Recurrent Herpes Simplex Virus Type 1 (HSV-1) Infection Modulates Neuronal Aging Marks in In Vitro and In Vivo Models.
Napoletani G, Protto V, Marcocci ME, Nencioni L, Palamara AT, De Chiara G. Napoletani G, et al. Int J Mol Sci. 2021 Jun 11;22(12):6279. doi: 10.3390/ijms22126279. Int J Mol Sci. 2021. PMID: 34208020 Free PMC article. - Interplay between Autophagy and Herpes Simplex Virus Type 1: ICP34.5, One of the Main Actors.
Ripa I, Andreu S, López-Guerrero JA, Bello-Morales R. Ripa I, et al. Int J Mol Sci. 2022 Nov 7;23(21):13643. doi: 10.3390/ijms232113643. Int J Mol Sci. 2022. PMID: 36362429 Free PMC article. Review. - Immunity in latent Herpes simplex virus infection.
Bystrická M, Russ G. Bystrická M, et al. Acta Virol. 2005;49(3):159-67. Acta Virol. 2005. PMID: 16178513 Review.
Cited by
- Nonstructural protein 6 of porcine epidemic diarrhea virus induces autophagy to promote viral replication via the PI3K/Akt/mTOR axis.
Lin H, Li B, Liu M, Zhou H, He K, Fan H. Lin H, et al. Vet Microbiol. 2020 May;244:108684. doi: 10.1016/j.vetmic.2020.108684. Epub 2020 Apr 18. Vet Microbiol. 2020. PMID: 32402351 Free PMC article. - A ravenous defense: canonical and non-canonical autophagy in immunity.
Sil P, Muse G, Martinez J. Sil P, et al. Curr Opin Immunol. 2018 Feb;50:21-31. doi: 10.1016/j.coi.2017.10.004. Epub 2017 Nov 7. Curr Opin Immunol. 2018. PMID: 29125936 Free PMC article. Review. - Herpes Simplex Virus Type 1 Infection of the Central Nervous System: Insights Into Proposed Interrelationships With Neurodegenerative Disorders.
Duarte LF, Farías MA, Álvarez DM, Bueno SM, Riedel CA, González PA. Duarte LF, et al. Front Cell Neurosci. 2019 Feb 26;13:46. doi: 10.3389/fncel.2019.00046. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30863282 Free PMC article. Review. - Disrupting Neurons and Glial Cells Oneness in the Brain-The Possible Causal Role of Herpes Simplex Virus Type 1 (HSV-1) in Alzheimer's Disease.
Mielcarska MB, Skowrońska K, Wyżewski Z, Toka FN. Mielcarska MB, et al. Int J Mol Sci. 2021 Dec 27;23(1):242. doi: 10.3390/ijms23010242. Int J Mol Sci. 2021. PMID: 35008671 Free PMC article. Review. - Molecular Mechanisms for Herpes Simplex Virus Type 1 Pathogenesis in Alzheimer's Disease.
Harris SA, Harris EA. Harris SA, et al. Front Aging Neurosci. 2018 Mar 6;10:48. doi: 10.3389/fnagi.2018.00048. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29559905 Free PMC article. Review.
References
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis 2014; 209:325-33; PMID:24136792; http://dx.doi.org/10.1093/infdis/jit458 - DOI - PubMed
- Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J Infect Dis 2002; 185:1019-24; PMID:11930310; http://dx.doi.org/10.1086/340041 - DOI - PubMed
- Wilson AC, Mohr I. A cultured affair: HSV latency and reactivation in neurons. Trends Microbiol 2012; 20:604-11; PMID:22963857; http://dx.doi.org/10.1016/j.tim.2012.08.005 - DOI - PMC - PubMed
- Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis 1998; 26:541-53; quiz 54-5; PMID:9524821; http://dx.doi.org/10.1086/514600 - DOI - PubMed
- Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, Gabrielli L, Licastro F. Herpes virus in Alzheimer disease: relation to progression of the disease. Neurobiol Aging 2014; 35:122-9; PMID:23916950; http://dx.doi.org/10.1016/j.neurobiolaging.2013.06.024 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical