Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy - PubMed (original) (raw)
Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy
Antonio Piras et al. Acta Neuropathol Commun. 2016.
Abstract
Introduction: The accumulation of insoluble proteins within neurons and glia cells is a pathological hallmark of several neurodegenerative diseases. Abnormal aggregation of the microtubule-associated protein tau characterizes the neuropathology of tauopathies, such as Alzheimer disease (AD), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP). An impairment of the lysosomal degradation pathway called macroautophagy, hereafter referred to as autophagy, could contribute to the accumulation of aggregated proteins. The role of autophagy in neurodegeneration has been intensively studied in the context of AD but there are few studies in other tauopathies and it is not known if defects in autophagy is a general feature of tauopathies. In the present study, we analysed autophagic and lysosomal markers in human post-mortem brain samples from patients with early-onset familial AD (FAD) with the APP Swedish mutation (APPswe), CBD and PSP and control individuals.
Results: FAD, CBD and PSP patients displayed an increase in LC3-positive vesicles in frontal cortex, indicating an accumulation of autophagic vesicles. Moreover, using double-immunohistochemistry and in situ proximity ligation assay, we observed colocalization of hyperphosphorylated tau with the autophagy marker LC3 in FAD, CBD and PSP patients but not in control individuals. Increased levels of the lysosomal marker LAMP1 was detected in FAD and CBD, and in addition Cathepsin D was diffusely spread in the cytoplasm in all tauopathies suggesting an impaired lysosomal integrity.
Conclusion: Taken together, our results indicate an accumulation of autophagic and lysosomal markers in human brain tissue from patients with primary tauopathies (CBD and PSP) as well as FAD, suggesting a defect of the autophagosome-lysosome pathway that may contribute to the development of tau pathology.
Figures
Fig. 1
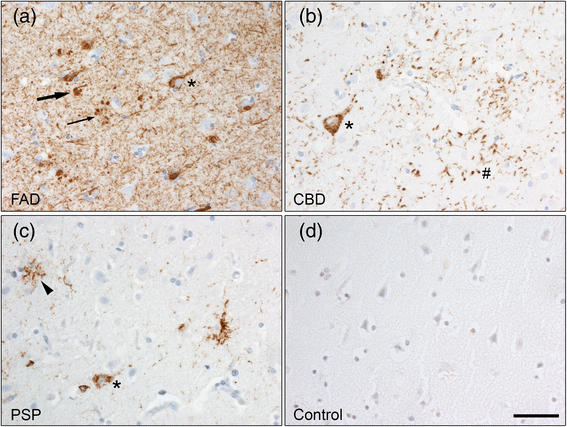
Tau pathology in frontal cortex as showed with the AT8 antibody. a APPswe mutation carriers show AT8 immunoreactivity in neurofibrillary tangles (arrow), pretangles (asterisk), neuropil threads and dystrophic neurites around amyloid plaques (small arrow). b AT8 immunohistochemistry revealed diffuse cytoplasmic tau immunoreactivity in neurons (pretangles, asterisk) and astrocytic plaques (#) in CBD patients, while c PSP patients show AT8 immunoreactivity in pretangles (asterisk) and tufted astrocytes (arrowhead). d No AT8 immunoreactivity was detected in control individuals. Scale bar: 50 μm
Fig. 2
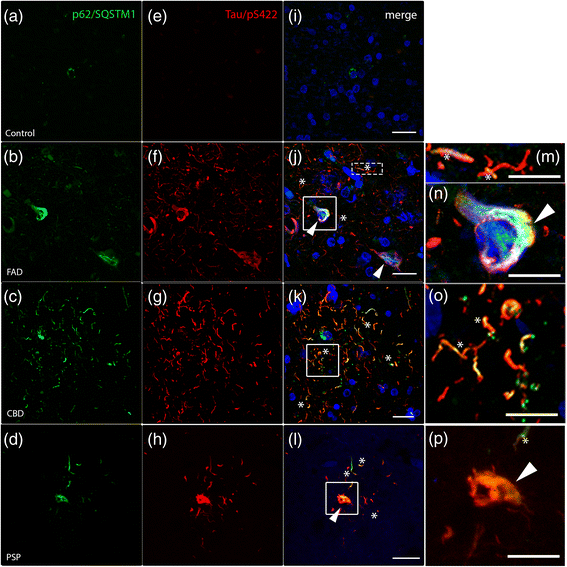
The autophagic marker p62/SQSTM1 accumulates and colocalizes with hyperphosphorylated tau in human tauopathies. Double immunohistochemistry against p62/SQSTM1 (green), hyperphosphorylated tau (Tau/pS422, red) and DAPI (blue) are shown by confocal analysis. a In control individuals, p62/SQSTM1-positive inclusions are only rarely observed and (e, i) no Tau/pS422 immunoreactivity were detected. b-h Strong immunoreactivity of p62/SQSTM1 (b-d) and Tau/pS422 (f-h) in post-mortem brain sections from FAD, CBD and PSP patients. j-l Merged pictures show colocalization between p62/SQSTM1 and Tau/pS422. j In FAD patients, colocalization is shown in neuronal threads (asterisks and high magnification m) and close to the nucleus (arrowheads and high magnification n). k, o In CBD patients, asterisks indicate colocalization in threads. l, p In PSP brains, colocalization is detected in threads (asterisks) and in some cell bodies (arrowheads). Scale: 20 μm (10 μm insert)
Fig. 3
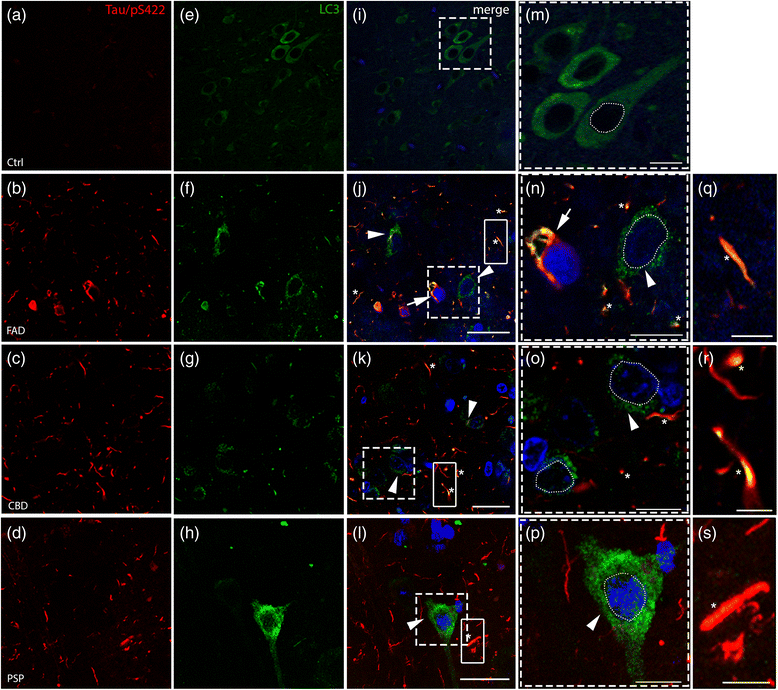
Accumulation of autophagic marker (LC3) and colocalization with hyperphosphorylated tau (Tau/pS422). Double immunofluorescence analysis on post-mortem tissues against (a-d) Tau/pS422 (red) and (e-h) LC3 (green). j-p Nuclei are stained with DAPI (blue, outlined with a white dashed line) in the merged pictures. e In control samples, LC3 staining is diffuse in the cytoplasm and LC3-positive dots are rarely observed (high magnification in (m) of the boxed area in i). j-l Arrowheads (and high magnifications, n-p) indicate cells showing accumulation of LC3 positive puncta in the perinuclear cytoplasm in FAD, CBD and PSP patients. Asterisks show colocalization in threads. In addition, FAD shows colocalization in tangle-like structures (j, n, arrows, see also SF. 2). q-s High magnification of the boxed areas in j-l showing colocalization of Tau/pS422 and LC3 in threads, respectively in FAD, CBD and PSP. Scale bars indicate 50 μm (i) and 25 μm (j-l); high magnification 10 μm (m-p) and 5 μm (q-s)
Fig. 4
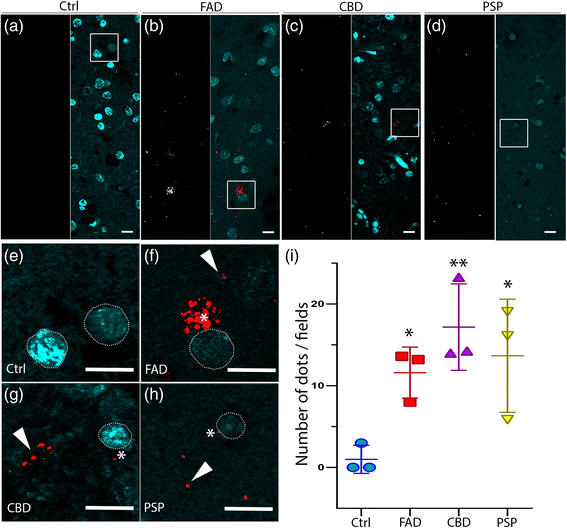
In situ PLA on human brain tissue confirms colocalization between hyperphosphorylated tau (AT8) and LC3. a-d The white signal shows the PLA dots in healthy controls and patients, respectively. In merged pictures, PLA staining (red) and DAPI (blue) are shown in FAD, CBD and PSP. PLA dots are rarely observed in control individuals. e-h High magnification of the boxed area from the merged pictures. Asterisks show PLA signal close to the nucleus (white dashed line), and arrowheads indicate PLA signal distant from clearly visible cell soma in FAD, CBD and PSP patients. Scale: 10 μm. i In the graphs PLA signal (number of dots/field) are indicated as Mean value ± S.D. from healthy controls and patients
Fig. 5
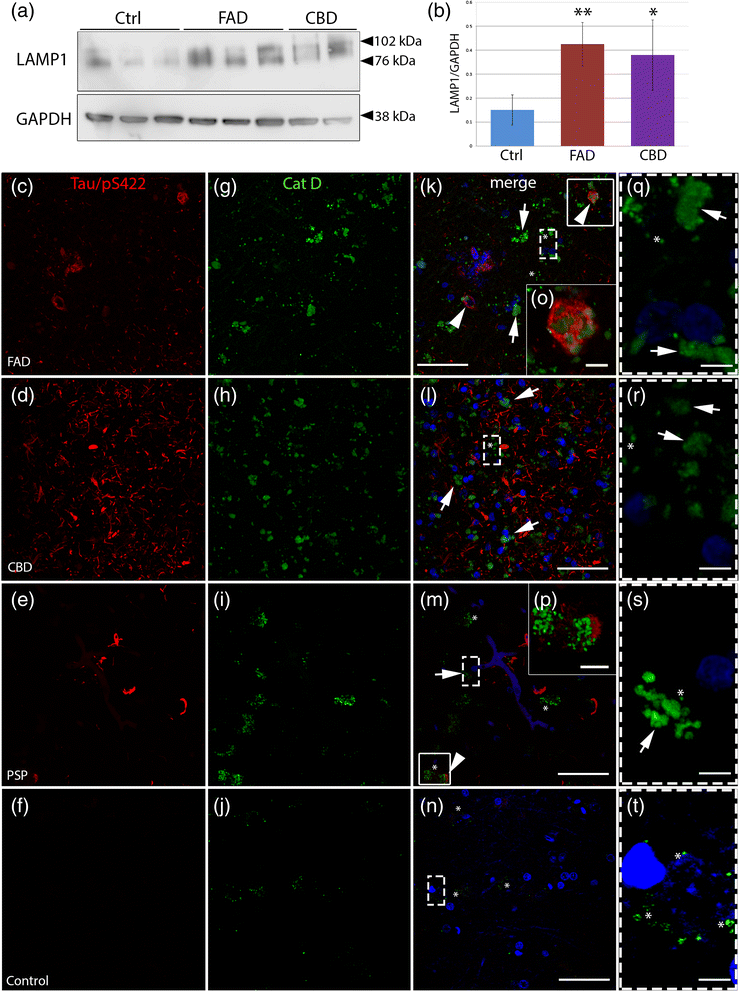
Accumulation of lysosomal markers and diffuse cytoplasmic Cat D immunoreactivity in tauopathies. a Western blot analysis of LAMP1 in human post-mortem brain (frontal cortex) extracts from control individuals (Ctrl, n = 3), early onset familial AD (FAD, n = 3) and CBD (n = 2). b LAMP1 levels quantified by densitometry and normalized to GAPDH in Ctrl, FAD and CBD. Results are shown as the mean value of arbitrary unit ± S.D (mean of three independent experiments). c-t Double immunohistochemistry against hyperphosphorylated tau (Tau/pS422, red, c-f) and Cat D (HPA003001, Sigma-Aldrich) (green, g-j) are shown by confocal analysis. k-n Merged pictures with nuclear staining (DAPI, blue). In FAD, CBD and PSP cases, strong Cat D-immunoreactivity is present in the soma of the cells in frontal cortex compared to the control. k-m (and high magnification q-s) Distinct vesicle-like structures (asterisks) and diffuse immunoreactivity (arrows) of Cat D throughout the cell bodies are shown in the pictures. In addition, in FAD and PSP, arrowheads in k and m and high magnification pictures (o-p) of boxed areas show hyperphosphorylated tau-positive structures close to Cat D-positive structures. t Brain tissues from control individuals show few Cat D-positive vesicle structures (asterisks). Scale bars: 50 μm (5 μm high magnification)
Similar articles
- Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal positioning and MAPT/Tau behavior during MAPT/Tau-induced proteotoxic stress.
Chambraud B, Daguinot C, Guillemeau K, Genet M, Dounane O, Meduri G, Poüs C, Baulieu EE, Giustiniani J. Chambraud B, et al. Autophagy. 2021 Nov;17(11):3491-3510. doi: 10.1080/15548627.2021.1875611. Epub 2021 Jan 25. Autophagy. 2021. PMID: 33459145 Free PMC article. - Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.
Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Viñals F, Ribalta T. Ferrer I, et al. Brain Pathol. 2001 Apr;11(2):144-58. doi: 10.1111/j.1750-3639.2001.tb00387.x. Brain Pathol. 2001. PMID: 11303790 Free PMC article. - Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration.
Yoshida M. Yoshida M. Neuropathology. 2014 Dec;34(6):555-70. doi: 10.1111/neup.12143. Epub 2014 Aug 14. Neuropathology. 2014. PMID: 25124031 Review. - Tau Interacting Proteins: Gaining Insight into the Roles of Tau in Health and Disease.
Stancu IC, Ferraiolo M, Terwel D, Dewachter I. Stancu IC, et al. Adv Exp Med Biol. 2019;1184:145-166. doi: 10.1007/978-981-32-9358-8_13. Adv Exp Med Biol. 2019. PMID: 32096036 Review.
Cited by
- Cellular and pathological functions of tau.
Parra Bravo C, Naguib SA, Gan L. Parra Bravo C, et al. Nat Rev Mol Cell Biol. 2024 Nov;25(11):845-864. doi: 10.1038/s41580-024-00753-9. Epub 2024 Jul 16. Nat Rev Mol Cell Biol. 2024. PMID: 39014245 Review. - Biomedical Implications of Autophagy in Macromolecule Storage Disorders.
Palhegyi AM, Seranova E, Dimova S, Hoque S, Sarkar S. Palhegyi AM, et al. Front Cell Dev Biol. 2019 Sep 6;7:179. doi: 10.3389/fcell.2019.00179. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31555645 Free PMC article. Review. - Vps10-mediated targeting of Pep4 determines the activity of the vacuole in a substrate-dependent manner.
Boutouja F, Stiehm CM, Mastalski T, Brinkmeier R, Reidick C, El Magraoui F, Platta HW. Boutouja F, et al. Sci Rep. 2019 Jul 22;9(1):10557. doi: 10.1038/s41598-019-47184-7. Sci Rep. 2019. PMID: 31332264 Free PMC article. - Impairment of the autophagy-lysosomal pathway in Alzheimer's diseases: Pathogenic mechanisms and therapeutic potential.
Zhang W, Xu C, Sun J, Shen HM, Wang J, Yang C. Zhang W, et al. Acta Pharm Sin B. 2022 Mar;12(3):1019-1040. doi: 10.1016/j.apsb.2022.01.008. Epub 2022 Jan 21. Acta Pharm Sin B. 2022. PMID: 35530153 Free PMC article. Review. - The potential role of glial cells in driving the prion-like transcellular propagation of tau in tauopathies.
Amro Z, Yool AJ, Collins-Praino LE. Amro Z, et al. Brain Behav Immun Health. 2021 Mar 17;14:100242. doi: 10.1016/j.bbih.2021.100242. eCollection 2021 Jul. Brain Behav Immun Health. 2021. PMID: 34589757 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous