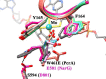Perchlorate Reductase Is Distinguished by Active Site Aromatic Gate Residues - PubMed (original) (raw)
Perchlorate Reductase Is Distinguished by Active Site Aromatic Gate Residues
Matthew D Youngblut et al. J Biol Chem. 2016.
Abstract
Perchlorate is an important ion on both Earth and Mars. Perchlorate reductase (PcrAB), a specialized member of the dimethylsulfoxide reductase superfamily, catalyzes the first step of microbial perchlorate respiration, but little is known about the biochemistry, specificity, structure, and mechanism of PcrAB. Here we characterize the biophysics and phylogeny of this enzyme and report the 1.86-Å resolution PcrAB complex crystal structure. Biochemical analysis revealed a relatively high perchlorate affinity (Km = 6 μm) and a characteristic substrate inhibition compared with the highly similar respiratory nitrate reductase NarGHI, which has a relatively much lower affinity for perchlorate (Km = 1.1 mm) and no substrate inhibition. Structural analysis of oxidized and reduced PcrAB with and without the substrate analog SeO3 (2-) bound to the active site identified key residues in the positively charged and funnel-shaped substrate access tunnel that gated substrate entrance and product release while trapping transiently produced chlorate. The structures suggest gating was associated with shifts of a Phe residue between open and closed conformations plus an Asp residue carboxylate shift between monodentate and bidentate coordination to the active site molybdenum atom. Taken together, structural and mutational analyses of gate residues suggest key roles of these gate residues for substrate entrance and product release. Our combined results provide the first detailed structural insight into the mechanism of biological perchlorate reduction, a critical component of the chlorine redox cycle on Earth.
Keywords: DMSO reductase superfamily; Mo-bis-MGD; enzyme mechanism; iron-sulfur protein; molybdenum; perchlorate reductase; structure-function; substrate access tunnel; substrate inhibition; x-ray crystallography.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
FIGURE 1.
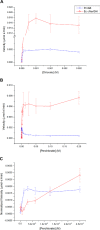
Steady-state kinetic curves for PcrAB and _Ec_cNarGHI for chlorate (A), perchlorate (B), and perchlorate (C) at low substrate concentrations (<200 μm). Error bars represent one standard deviation of triplicate assays.
FIGURE 2.
Crystal structure of oxidized PcrAB heterodimer reveals [Fe-S] clusters are 10–12 Å apart from the adjacent cluster and the Mo-bis-MGD cofactor. A, overall crystal structure of oxidized PcrAB. PcrA and PcrB are shown in green and cyan ribbon, respectively. [Fe-S] clusters are shown in sphere (iron, brown; sulfur, yellow), bis-MGD cofactor is shown in magenta ball-and-stick, and molybdenum is shown in turquoise sphere. B, the relative position of cofactors in PcrAB along with Asp170 and a water molecule that coordinate to the active site molybdenum atom. Edge-to-edge distances between cofactors are shown. Labeling of the [Fe-S] clusters is consistent with previous publications (35, 36). The tricyclic pyranopterin of the molybdopterin cofactor is labeled as MGD, and the bicyclic dihydropterin is labeled as MD1. C, electron density 2|Fo| − |Fc| simulated annealing omit maps (gray) at 1.5 σ contour level of PcrA cofactors and active site for oxidized PcrAB. The tricyclic pyranopterin of the molybdopterin cofactor is labeled as MGD, and the bicyclic dihydropterin is labeled as MD1.
FIGURE 3.
Comparisons of substrate accessing tunnels between PcrA and NarG identify the aromatic gate residues and the differences in electrostatic potential. A, structure of the closed tunnel in PcrA from the surface of the enzyme to the active site as seen in the oxidized PcrAB crystal structure (Fig. 2_A_) forms a funnel shape (cyan). The surface of the closed tunnel was detected by CASTp (40) and depicted using Chimera (25). The gate residues Phe164 (red), Tyr165 (orange), and Trp461 (orange) are shown in stick. B, the electrostatic potential surface of PcrA. The figure is viewed from the mouth of the tunnel down to the active site. The color is shown from negative (red; −10 kcal/mol × e) through white to positive (blue; 10 kcal/mol × e). C, structure of closed tunnel (blue) in _Ec_cNarG (Protein Data Bank code 1Q16) (35) forms a wider tunnel (tunnel volume, 1682 Å3) toward the active site than the tunnel in PcrA (tunnel volume, 1089 Å3). The corresponding gate residues in NarG are shown in stick with the same color code. D, the electrostatic potential surface of NarG by viewing from the same angle as B.
FIGURE 4.
Phylogenetic reconstruction of PcrA and closely related DMSO reductase superfamily enzymes using DmsA and DorA as an outgroup. Active site residues identified from the crystal structure of PcrA were visualized for clades with high bootstrap support using sequence motifs. The positions denoted by α, β, and δ in the sequence alignments correspond to the gate residues relative to the A. suillum PS PcrA. The positions denoted by γ in the sequence alignments correspond to the Asp residue that has been shown or is predicted to bind to the molybdenum atom at the active site of the corresponding enzyme. N and C represent the N- and C-terminal portions of the MBD in each enzyme. The fraction of predicted twin arginine translocation (TAT) signal sequences, physiological function of known members, and taxonomic affiliation are superimposed on the tree. For the clades containing SerA, ClrA, and EbdA, the darker highlighting in the C-terminal portion of the aligned sequences denotes lower confidence in the alignment. SerA is selenate reductase, ClrA is chlorate reductase, EbdA is ethylbenzene dehydrogenase, PcrA is perchlorate reductase, pNarG is periplasmic nitrate reductase, and cNarG is cytoplasmic nitrate reductase. A horizontal dash in the lowermost cNar clade denotes the compression of this large group of sequences into a group suitable for visualization.
FIGURE 5.
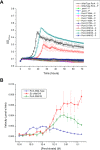
A, growth curves for wild-type A. suillum PS, Δ_pcrA_, and PcrA mutant strains under perchlorate or chlorate reducing conditions. C is chlorate, and P is perchlorate. Error bars represent one standard deviation of triplicate growth experiments. B, steady-state kinetics of perchlorate reduction by PcrAB containing the PcrA W461E mutation in comparison with wild-type PcrAB and _Ec_NarGHI. Error bars represent one standard deviation of triplicate assays.
FIGURE 6.
The comparison of PcrA W461E and _Ec_cNarG (Protein Data Bank code 1Q16) in the substrate access tunnel. The overlay of residue W461E PcrA from six chains of PcrA in the asymmetric unit of PcrAB was shown to be highly flexible due to the lack of hydrogen binding with Ser594 and the loss of π-stacking with Tyr165. The corresponding residue Glu581 in _Ec_cNarG (magenta) was stabilized by residue Asp801 via hydrogen bonding. The wild-type PcrAB is shown in dark gray color with Trp461 stabilized by π-stacking with Tyr165.
FIGURE 7.
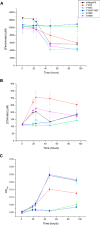
Chlorate accumulation experiments of wild-type A. suillum PS and A. suillum PS cells containing pcrA mutations monitoring chlorate (A), perchlorate (B), and OD600 (C). Error bars represent the standard deviation of three replicate experiments.
FIGURE 8.
Reduced PcrAB structures with substrate analog bound and open and closed conformation of PcrA Phe164. A, active site of PcrA (blue) with selenite (SeO32−) coordinated to the molybdenum atom. The occupancy of selenite in the structure is 0.6. The surfaces in A and C are colored in a hydrophobicity scale (26) where red is the most hydrophobic and blue is the most hydrophilic. B, electron density of 2|Fo| − |Fc| simulated annealing omit maps at 1.5 σ contour level of PcrA cofactors and active site for reduced PcrAB with selenite bound. The anomalous map is shown as a green surface at 3 σ contour level. C, overlay of the active sites of oxidized (magenta) and reduced (tan) PcrA along with the tunnel to the active site. The reduced PcrA structure reveals dual conformations of the Phe164 side chain in open and closed states. The tunnel is shown in the open conformation of Phe164. The water molecule in reduced PcrA (tan) is located inside the open tunnel and hydrogen-bonded with residues Asp170 and Asn35. D, electron density of 2|Fo| − |Fc| simulated annealing omit maps at 1.5 σ contour level of PcrA cofactors and active site for reduced PcrAB with the side chain of Phe164 in the open and closed conformations. E, structure of the open tunnel in PcrA with Phe164 in the open tunnel conformation shown as a surface (light purple).
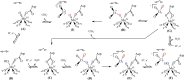
FIGURE 9.
Proposed mechanism for (per)chlorate reduction by PcrAB (A–I). The Asp residue corresponds to Asp170 as shown in the oxidized and reduced crystal structures of PcrAB. The symbols for a closed or an open switch represent a closed or open gate, respectively.
Similar articles
- An uncharacterized clade in the DMSO reductase family of molybdenum oxidoreductases is a new type of chlorate reductase.
Barnum TP, Coates JD. Barnum TP, et al. Environ Microbiol Rep. 2020 Oct;12(5):534-539. doi: 10.1111/1758-2229.12869. Epub 2020 Aug 10. Environ Microbiol Rep. 2020. PMID: 32627393 - Identification, characterization, and classification of genes encoding perchlorate reductase.
Bender KS, Shang C, Chakraborty R, Belchik SM, Coates JD, Achenbach LA. Bender KS, et al. J Bacteriol. 2005 Aug;187(15):5090-6. doi: 10.1128/JB.187.15.5090-5096.2005. J Bacteriol. 2005. PMID: 16030201 Free PMC article. - Mechanism of H2S Oxidation by the Dissimilatory Perchlorate-Reducing Microorganism Azospira suillum PS.
Mehta-Kolte MG, Loutey D, Wang O, Youngblut MD, Hubbard CG, Wetmore KM, Conrad ME, Coates JD. Mehta-Kolte MG, et al. mBio. 2017 Feb 21;8(1):e02023-16. doi: 10.1128/mBio.02023-16. mBio. 2017. PMID: 28223460 Free PMC article. - Microbial respiration with chlorine oxyanions: diversity and physiological and biochemical properties of chlorate- and perchlorate-reducing microorganisms.
Liebensteiner MG, Oosterkamp MJ, Stams AJ. Liebensteiner MG, et al. Ann N Y Acad Sci. 2016 Feb;1365(1):59-72. doi: 10.1111/nyas.12806. Epub 2015 Jun 23. Ann N Y Acad Sci. 2016. PMID: 26104311 Review. - Ethylbenzene Dehydrogenase and Related Molybdenum Enzymes Involved in Oxygen-Independent Alkyl Chain Hydroxylation.
Heider J, Szaleniec M, Sünwoldt K, Boll M. Heider J, et al. J Mol Microbiol Biotechnol. 2016;26(1-3):45-62. doi: 10.1159/000441357. Epub 2016 Mar 10. J Mol Microbiol Biotechnol. 2016. PMID: 26960184 Review.
Cited by
- Genome-resolved metagenomics identifies genetic mobility, metabolic interactions, and unexpected diversity in perchlorate-reducing communities.
Barnum TP, Figueroa IA, Carlström CI, Lucas LN, Engelbrektson AL, Coates JD. Barnum TP, et al. ISME J. 2018 Jun;12(6):1568-1581. doi: 10.1038/s41396-018-0081-5. Epub 2018 Feb 23. ISME J. 2018. PMID: 29476141 Free PMC article. - Making Moco: A Personal History.
Burgmayer SJN. Burgmayer SJN. Molecules. 2023 Oct 27;28(21):7296. doi: 10.3390/molecules28217296. Molecules. 2023. PMID: 37959716 Free PMC article. Review. - Functional Redundancy in Perchlorate and Nitrate Electron Transport Chains and Rewiring Respiratory Pathways to Alter Terminal Electron Acceptor Preference.
Wang O, Melnyk RA, Mehta-Kolte MG, Youngblut MD, Carlson HK, Coates JD. Wang O, et al. Front Microbiol. 2018 Mar 6;9:376. doi: 10.3389/fmicb.2018.00376. eCollection 2018. Front Microbiol. 2018. PMID: 29559962 Free PMC article. - Integration of molecular and computational approaches paints a holistic portrait of obscure metabolisms.
Reyes-Umana V, Ewens SD, Meier DAO, Coates JD. Reyes-Umana V, et al. mBio. 2023 Dec 19;14(6):e0043123. doi: 10.1128/mbio.00431-23. Epub 2023 Oct 19. mBio. 2023. PMID: 37855625 Free PMC article. Review. - Oxygen Generation via Water Splitting by a Novel Biogenic Metal Ion-Binding Compound.
Dershwitz P, Bandow NL, Yang J, Semrau JD, McEllistrem MT, Heinze RA, Fonseca M, Ledesma JC, Jennett JR, DiSpirito AM, Athwal NS, Hargrove MS, Bobik TA, Zischka H, DiSpirito AA. Dershwitz P, et al. Appl Environ Microbiol. 2021 Jun 25;87(14):e0028621. doi: 10.1128/AEM.00286-21. Epub 2021 Jun 25. Appl Environ Microbiol. 2021. PMID: 33962982 Free PMC article.
References
- Jackson W. A., Bohlke J. K., Andraski B. J., Fahlquist L., Bexfield L., Eckardt F. D., Gates J. B., Davila A. F., McKay C. P., Rao B., Sevanthi R., Rajagopalan S., Estrada N., Sturchio N., Hatzinger P. B., Anderson T. A., Orris G., Betancourt J., Stonestrom D., Latorre C., Li Y. H., and Harvey G. J. (2015) Global patterns and environmental controls of perchlorate and nitrate co-occurrence in arid and semi-arid environments. Geochim. Cosmochim. Acta 164, 502–522
- Jackson W. A., Davila A. F., Sears D. W. G., Coates J. D., Mckay C. P., Brundrett M., Estrada N., and Bohlke J. K. (2015) Widespread occurrence of (per)chlorate in the solar system. Earth Planet. Sci. Lett. 430, 470–476
- Coates J. D., and Achenbach L. A. (2004) Microbial perchlorate reduction: rocket-fuelled metabolism. Nat. Rev. Microbiol. 2, 569–580 - PubMed
- Rajagopalan S., Anderson T., Cox S., Harvey G., Cheng Q., and Jackson W. A. (2009) Perchlorate in wet deposition across North America. Environ. Sci. Technol. 43, 616–622 - PubMed
- Hecht M. H., Kounaves S. P., Quinn R. C., West S. J., Young S. M., Ming D. W., Catling D. C., Clark B. C., Boynton W. V., Hoffman J., Deflores L. P., Gospodinova K., Kapit J., and Smith P. H. (2009) Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 325, 64–67 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous