Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial - PubMed (original) (raw)
Clinical Trial
. 2016 May 7;387(10031):1909-20.
doi: 10.1016/S0140-6736(16)00561-4. Epub 2016 Mar 4.
Jean Hoffman-Censits 2, Tom Powles 3, Michiel S van der Heijden 4, Arjun V Balar 5, Andrea Necchi 6, Nancy Dawson 7, Peter H O'Donnell 8, Ani Balmanoukian 9, Yohann Loriot 10, Sandy Srinivas 11, Margitta M Retz 12, Petros Grivas 13, Richard W Joseph 14, Matthew D Galsky 15, Mark T Fleming 16, Daniel P Petrylak 17, Jose Luis Perez-Gracia 18, Howard A Burris 19, Daniel Castellano 20, Christina Canil 21, Joaquim Bellmunt 22, Dean Bajorin 23, Dorothee Nickles 24, Richard Bourgon 24, Garrett M Frampton 25, Na Cui 24, Sanjeev Mariathasan 24, Oyewale Abidoye 24, Gregg D Fine 24, Robert Dreicer 26
Affiliations
- PMID: 26952546
- PMCID: PMC5480242
- DOI: 10.1016/S0140-6736(16)00561-4
Clinical Trial
Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial
Jonathan E Rosenberg et al. Lancet. 2016.
Abstract
Background: Patients with metastatic urothelial carcinoma have few treatment options after failure of platinum-based chemotherapy. In this trial, we assessed treatment with atezolizumab, an engineered humanised immunoglobulin G1 monoclonal antibody that binds selectively to programmed death ligand 1 (PD-L1), in this patient population.
Methods: For this multicentre, single-arm, two-cohort, phase 2 trial, patients (aged ≥18 years) with inoperable locally advanced or metastatic urothelial carcinoma whose disease had progressed after previous platinum-based chemotherapy were enrolled from 70 major academic medical centres and community oncology practices in Europe and North America. Key inclusion criteria for enrolment were Eastern Cooperative Oncology Group performance status of 0 or 1, measurable disease defined by Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1), adequate haematological and end-organ function, and no autoimmune disease or active infections. Formalin-fixed paraffin-embedded tumour specimens with sufficient viable tumour content were needed from all patients before enrolment. Patients received treatment with intravenous atezolizumab (1200 mg, given every 3 weeks). PD-L1 expression on tumour-infiltrating immune cells (ICs) was assessed prospectively by immunohistochemistry. The co-primary endpoints were the independent review facility-assessed objective response rate according to RECIST v1.1 and the investigator-assessed objective response rate according to immune-modified RECIST, analysed by intention to treat. A hierarchical testing procedure was used to assess whether the objective response rate was significantly higher than the historical control rate of 10% at an α level of 0·05. This study is registered with ClinicalTrials.gov, number NCT02108652.
Findings: Between May 13, 2014, and Nov 19, 2014, 486 patients were screened and 315 patients were enrolled into the study. Of these patients, 310 received atezolizumab treatment (five enrolled patients later did not meet eligibility criteria and were not dosed with study drug). The PD-L1 expression status on infiltrating immune cells (ICs) in the tumour microenvironment was defined by the percentage of PD-L1-positive immune cells: IC0 (<1%), IC1 (≥1% but <5%), and IC2/3 (≥5%). The primary analysis (data cutoff May 5, 2015) showed that compared with a historical control overall response rate of 10%, treatment with atezolizumab resulted in a significantly improved RECIST v1.1 objective response rate for each prespecified immune cell group (IC2/3: 27% [95% CI 19-37], p<0·0001; IC1/2/3: 18% [13-24], p=0·0004) and in all patients (15% [11-20], p=0·0058). With longer follow-up (data cutoff Sept 14, 2015), by independent review, objective response rates were 26% (95% CI 18-36) in the IC2/3 group, 18% (13-24) in the IC1/2/3 group, and 15% (11-19) overall in all 310 patients. With a median follow-up of 11·7 months (95% CI 11·4-12·2), ongoing responses were recorded in 38 (84%) of 45 responders. Exploratory analyses showed The Cancer Genome Atlas (TCGA) subtypes and mutation load to be independently predictive for response to atezolizumab. Grade 3-4 treatment-related adverse events, of which fatigue was the most common (five patients [2%]), occurred in 50 (16%) of 310 treated patients. Grade 3-4 immune-mediated adverse events occurred in 15 (5%) of 310 treated patients, with pneumonitis, increased aspartate aminotransferase, increased alanine aminotransferase, rash, and dyspnoea being the most common. No treatment-related deaths occurred during the study.
Interpretation: Atezolizumab showed durable activity and good tolerability in this patient population. Increased levels of PD-L1 expression on immune cells were associated with increased response. This report is the first to show the association of TCGA subtypes with response to immune checkpoint inhibition and to show the importance of mutation load as a biomarker of response to this class of agents in advanced urothelial carcinoma.
Funding: F Hoffmann-La Roche Ltd.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Trial profile for Cohort 2
*Based on May 5, 2015 data cut. Two Cohort 2 patients and one Cohort 1 patient were re-assigned to the alternate cohort based on eligibility reassessments between the May 5 and September 14, 2015 data cuts (enrolled and treated n’s based on September data cut are 315 and 310, respectively). ‡Excludes 1 patient with unknown site. † Includes rescreened patients. ECOG PS, Eastern Cooperative Oncology Group performance status; ICF, informed consent form; UC, urothelial carcinoma.
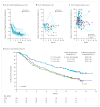
Figure 2. Change in sum of longest diameters over time by best response in the PD-L1 IC2/3 group and comparison of overall survival among PD-L1 IC groups
Percent change in the sum of longest diameters (SLD) by independent review assessed RECIST v1.1 in the IC2/3 group by (A) responders; (B) stable disease; (C) progressive disease. Patients without a measurable baseline tumor assessment or without post-baseline tumor measurements were not included. (D) Kaplan-Meier overall survival curves for the IC0, IC1, and IC2/3 groups. IC, immune cell; CI, confidence interval.
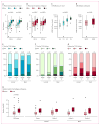
Figure 3. Association of response and PD-L1 IHC status with gene expression profiling and mutation load
(A) Association of PD-L1 IHC IC with gene expression for CXCL9 and CXCL10, two representatives of a CD8 T effector gene set. For each gene, expression increased linearly with IC score (p<0·0001 and p<0·0001, respectively). The other genes in an eight-gene CD8 T effector gene set behaved similarly (figure 6A, appendix). (B) CXCL9 and CXCL10 were significantly associated with response (p=0·0057 and p=0·0079, respectively). The other genes in the set did not achieve statistical significance individually, likely due to lower RNA-seq read counts, but they exhibited behavior qualitatively similar to that of CXCL9 and CXCL10 (figure 6B, appendix). (C) Tumor CD8+ T cell infiltration in the tumor center was significantly associated with PD-L1 IC (p<0·0001). (D) Response was significantly associated with increased CD8+ IHC staining in the tumor center (p=0.0265). (E) IC score distribution by TCGA subtype. IC2/3 was significantly more common in the basal subtypes (III and IV, p<0·0001), though IC2 was present in all subtypes. (F) TC score distributions by TCGA subtype. TC2/3 was almost exclusively seen in basal subtypes (p<0·0001). (G) Objective response rate by TCGA subtype. Response was significantly associated with TCGA subtype: 10% for subtype I, 34% for II, 16% for III, and 20% for IV (p=0·0102). (H) Estimated mutation load per megabase (Mb) vs patient response, overall (n=150) and also disaggregated by TCGA subtype. Mutation load was strongly associated with response (p<0·0001), but to a similar degree in all TCGA subtypes. IC, immune cell; TC, tumor cell; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Comment in
- Role for anti-PD-L1 immune checkpoint inhibitor in advanced urothelial carcinoma.
Netto GJ. Netto GJ. Lancet. 2016 May 7;387(10031):1881-2. doi: 10.1016/S0140-6736(16)00654-1. Epub 2016 Mar 4. Lancet. 2016. PMID: 26952549 No abstract available. - Urological cancer: Atezolizumab effective against advanced disease.
Sidaway P. Sidaway P. Nat Rev Clin Oncol. 2016 May;13(5):266. doi: 10.1038/nrclinonc.2016.48. Epub 2016 Mar 22. Nat Rev Clin Oncol. 2016. PMID: 27000959 No abstract available. - Bladder cancer: Atezolizumab effective against advanced-stage disease.
Sidaway P. Sidaway P. Nat Rev Urol. 2016 May;13(5):238. doi: 10.1038/nrurol.2016.60. Epub 2016 Mar 22. Nat Rev Urol. 2016. PMID: 27001012 No abstract available. - Atezolizumab and bladder cancer: facing a complex disease.
Cattrini C, Boccardo F. Cattrini C, et al. Lancet. 2018 Jan 27;391(10118):305-306. doi: 10.1016/S0140-6736(18)30095-3. Epub 2018 Jan 31. Lancet. 2018. PMID: 29413038 No abstract available.
Similar articles
- Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A; POPLAR Study Group. Fehrenbacher L, et al. Lancet. 2016 Apr 30;387(10030):1837-46. doi: 10.1016/S0140-6736(16)00587-0. Epub 2016 Mar 10. Lancet. 2016. PMID: 26970723 Clinical Trial. - Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial.
Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, Fléchon A, Gravis G, Hussain S, Takano T, Leng N, Kadel EE 3rd, Banchereau R, Hegde PS, Mariathasan S, Cui N, Shen X, Derleth CL, Green MC, Ravaud A. Powles T, et al. Lancet. 2018 Feb 24;391(10122):748-757. doi: 10.1016/S0140-6736(17)33297-X. Epub 2017 Dec 18. Lancet. 2018. PMID: 29268948 Clinical Trial. - Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial.
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Durán I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE 3rd, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thåström A, Abidoye OO, Fine GD, Bajorin DF; IMvigor210 Study Group. Balar AV, et al. Lancet. 2017 Jan 7;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2. Epub 2016 Dec 8. Lancet. 2017. PMID: 27939400 Free PMC article. Clinical Trial. - [Atezolizumab (Tecentriq®): Activity, indication and modality of use in advanced or metastatic urinary bladder carcinoma].
Bernard-Tessier A, Bonnet C, Lavaud P, Gizzi M, Loriot Y, Massard C. Bernard-Tessier A, et al. Bull Cancer. 2018 Feb;105(2):140-145. doi: 10.1016/j.bulcan.2017.10.030. Epub 2017 Dec 28. Bull Cancer. 2018. PMID: 29290331 Review. French. - Atezolizumab in invasive and metastatic urothelial carcinoma.
Crist M, Balar A. Crist M, et al. Expert Rev Clin Pharmacol. 2017 Dec;10(12):1295-1301. doi: 10.1080/17512433.2017.1389275. Epub 2017 Oct 30. Expert Rev Clin Pharmacol. 2017. PMID: 28994323 Review.
Cited by
- Progress and Challenges of Predictive Biomarkers for Immune Checkpoint Blockade.
Lei Y, Li X, Huang Q, Zheng X, Liu M. Lei Y, et al. Front Oncol. 2021 Mar 11;11:617335. doi: 10.3389/fonc.2021.617335. eCollection 2021. Front Oncol. 2021. PMID: 33777757 Free PMC article. Review. - Coming of Age of Immunotherapy of Urothelial Cancer.
Grande E, Molina-Cerrillo J, Necchi A. Grande E, et al. Target Oncol. 2021 May;16(3):283-294. doi: 10.1007/s11523-021-00804-7. Epub 2021 Mar 12. Target Oncol. 2021. PMID: 33710533 Review. - Open-label, dose-escalation FIGHT-101 study of pemigatinib combined with targeted therapy, chemotherapy, or immunotherapy in patients with advanced malignancies.
Saleh M, Barve M, Subbiah V, Papadopoulos KP, Morgensztern D, Mettu NB, Roychowdhury S, Spanggaard I, Veronese ML, Tian C, Silverman IM, Gutierrez M. Saleh M, et al. ESMO Open. 2024 Jul;9(7):103625. doi: 10.1016/j.esmoop.2024.103625. Epub 2024 Jul 9. ESMO Open. 2024. PMID: 38986210 Free PMC article. Clinical Trial. - Comprehensive Molecular Characterization of Urachal Adenocarcinoma Reveals Commonalities With Colorectal Cancer, Including a Hypermutable Phenotype.
Kardos J, Wobker SE, Woods ME, Nielsen ME, Smith AB, Wallen EM, Pruthi RS, Hayward MC, McGinty KA, Grilley-Olson JE, Patel NM, Weck KE, Black P, Parker JS, Milowsky MI, Hayes DN, Kim WY. Kardos J, et al. JCO Precis Oncol. 2017 Jul 6;1:PO.17.00027. doi: 10.1200/PO.17.00027. eCollection 2017. JCO Precis Oncol. 2017. PMID: 32913973 Free PMC article. - Macrophage correlates with immunophenotype and predicts anti-PD-L1 response of urothelial cancer.
Zeng D, Ye Z, Wu J, Zhou R, Fan X, Wang G, Huang Y, Wu J, Sun H, Wang M, Bin J, Liao Y, Li N, Shi M, Liao W. Zeng D, et al. Theranostics. 2020 May 25;10(15):7002-7014. doi: 10.7150/thno.46176. eCollection 2020. Theranostics. 2020. PMID: 32550918 Free PMC article.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. - PubMed
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. - PubMed
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials