Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth - PubMed (original) (raw)
. 2016 May 5;18(5):587-90.
doi: 10.1016/j.stem.2016.02.016. Epub 2016 Mar 4.
Christy Hammack 2, Sarah C Ogden 2, Zhexing Wen 3, Xuyu Qian 4, Yujing Li 5, Bing Yao 5, Jaehoon Shin 6, Feiran Zhang 5, Emily M Lee 2, Kimberly M Christian 3, Ruth A Didier 7, Peng Jin 5, Hongjun Song 8, Guo-Li Ming 9
Affiliations
- PMID: 26952870
- PMCID: PMC5299540
- DOI: 10.1016/j.stem.2016.02.016
Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth
Hengli Tang et al. Cell Stem Cell. 2016.
Abstract
The suspected link between infection by Zika virus (ZIKV), a re-emerging flavivirus, and microcephaly is an urgent global health concern. The direct target cells of ZIKV in the developing human fetus are not clear. Here we show that a strain of the ZIKV, MR766, serially passaged in monkey and mosquito cells efficiently infects human neural progenitor cells (hNPCs) derived from induced pluripotent stem cells. Infected hNPCs further release infectious ZIKV particles. Importantly, ZIKV infection increases cell death and dysregulates cell-cycle progression, resulting in attenuated hNPC growth. Global gene expression analysis of infected hNPCs reveals transcriptional dysregulation, notably of cell-cycle-related pathways. Our results identify hNPCs as a direct ZIKV target. In addition, we establish a tractable experimental model system to investigate the impact and mechanism of ZIKV on human brain development and provide a platform to screen therapeutic compounds.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
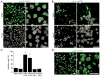
Figure 1. ZIKV Infects hiPSC-derived Neural Progenitor Cells with High Efficiency
(A–B) Sample confocal images of forebrain-specific hNPCs (A) and immature neurons (B) 56 hours after infection with ZIKV supernatant, and immunostained for ZIKV envelop protein (ZIKVE; green) and DAPI (gray). Cells were differentiated from the C1-2 hiPSC line. Scale bars: 20 μm. (C) Quantification of infection efficiency for different cell types, including hESCs, hiPSCs, hNPCS derived from two different hiPSCs, and immature neurons 1 or 9 days after differentiation from hNPCs. Both hESCs and hiPSCs were analyzed 72 hours after infection, whereas all other cells were analyzed 56 hours after infection. Numbers associated with bar graphs indicate numbers of independent experiments. Values represent mean ± SD (*P < 0.01; Student’s t-test) (D) Production of infectious ZIKV particles by infected hNPCs. Supernatant from hNPC cultures 72 hours after ZIKV infection was collected and added to Vero cells for 2 hours. The Vero cells were further cultured for 48 hours. Shown are sample images of ZIKVE immunostaining (green) and DAPI (gray). Scale bars: 20 μm. See also Figure S1 and Table S1
Figure 2. ZIKV-infected hNPCs Exhibit Increased Cell Death and Dysregulated Cell Cycle Progression and Gene Expression
(A–B) Increased cell death of ZIKV-infected hNPCs. Shown in (A) are sample images of immunostaining of hNPCs for ZIKVE (green) and cleaved-caspase 3 (Cas3; red) and DAPI (gray) 72 hours after ZIKV infection. Scale bars: 20 μm. Shown in (B) is the quantification. Values represent mean ± SEM (n = 6; *P < 0.01; Student’s t-test). (C) Cell cycle perturbation of hNPCs infected by ZIKV. Shown are sample flow cytometry analyses of distributions of hNPCs (from the C1-2 line) at different phases of cell cycle 72 hours after ZIKV or Mock infection. For the mixture sample, mock and infected hNPCs were mixed at a ratio of 1:1 following Propidium Iodide staining of each sample. (D–E) RNA-seq analysis of hNPCs (C1-2 line) 56 hours after ZIKV or mock infection. Genes with significant differences in expression between infected and uninfected hNPCs were subjected to GO analyses. The top 10 most significant terms are shown for downregulated (D) and upregulated (E) genes, respectively. The −log10 p-values are indicated by bar plots. An additional term of regulation of programmed cell death is also shown for upregulated genes (E). See also Figure S2 and Table S2
Comment in
- Understanding How Zika Virus Enters and Infects Neural Target Cells.
Miner JJ, Diamond MS. Miner JJ, et al. Cell Stem Cell. 2016 May 5;18(5):559-60. doi: 10.1016/j.stem.2016.04.009. Cell Stem Cell. 2016. PMID: 27152436 - Racing to Uncover the Link between Zika Virus and Microcephaly.
Ming GL, Song H, Tang H. Ming GL, et al. Cell Stem Cell. 2017 Jun 1;20(6):749-753. doi: 10.1016/j.stem.2017.05.010. Cell Stem Cell. 2017. PMID: 28575689
Similar articles
- Zika Virus Infection Induces DNA Damage Response in Human Neural Progenitors That Enhances Viral Replication.
Hammack C, Ogden SC, Madden JC Jr, Medina A, Xu C, Phillips E, Son Y, Cone A, Giovinazzi S, Didier RA, Gilbert DM, Song H, Ming G, Wen Z, Brinton MA, Gunjan A, Tang H. Hammack C, et al. J Virol. 2019 Sep 30;93(20):e00638-19. doi: 10.1128/JVI.00638-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375586 Free PMC article. - Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice.
Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Li C, et al. Cell Stem Cell. 2016 Jul 7;19(1):120-6. doi: 10.1016/j.stem.2016.04.017. Epub 2016 May 11. Cell Stem Cell. 2016. PMID: 27179424 - Zika virus differentially infects human neural progenitor cells according to their state of differentiation and dysregulates neurogenesis through the Notch pathway.
Ferraris P, Cochet M, Hamel R, Gladwyn-Ng I, Alfano C, Diop F, Garcia D, Talignani L, Montero-Menei CN, Nougairède A, Yssel H, Nguyen L, Coulpier M, Missé D. Ferraris P, et al. Emerg Microbes Infect. 2019;8(1):1003-1016. doi: 10.1080/22221751.2019.1637283. Emerg Microbes Infect. 2019. PMID: 31282298 Free PMC article. - The impact of Zika virus in the brain.
Russo FB, Beltrão-Braga PCB. Russo FB, et al. Biochem Biophys Res Commun. 2017 Oct 28;492(4):603-607. doi: 10.1016/j.bbrc.2017.01.074. Epub 2017 Jan 17. Biochem Biophys Res Commun. 2017. PMID: 28108286 Review. - Zika infection and the development of neurological defects.
Russo FB, Jungmann P, Beltrão-Braga PCB. Russo FB, et al. Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review.
Cited by
- AMFR-mediated Flavivirus NS2A ubiquitination subverts ER-phagy to augment viral pathogenicity.
Zhang L, Wang H, Han C, Dong Q, Yan J, Guo W, Shan C, Zhao W, Chen P, Huang R, Wu Y, Chen Y, Qin Y, Chen M. Zhang L, et al. Nat Commun. 2024 Nov 6;15(1):9578. doi: 10.1038/s41467-024-54010-w. Nat Commun. 2024. PMID: 39505910 Free PMC article. - The mechanisms of Zika virus-induced neuropathogenesis.
Hassaan NA, Xing L. Hassaan NA, et al. Braz J Microbiol. 2024 Oct 18. doi: 10.1007/s42770-024-01543-3. Online ahead of print. Braz J Microbiol. 2024. PMID: 39422868 Review. - The Human Neural Cell Atlas of Zika Infection in developing human brain tissue: viral pathogenesis, innate immunity, and lineage reprogramming.
Stokes C, Whitmore LS, Moreno D, Malhotra K, Tisoncik-Go J, Tran E, Wren N, Glass I; Birth Defects Research Laboratory (BDRL); Young JE, Gale M Jr. Stokes C, et al. bioRxiv [Preprint]. 2024 Sep 29:2024.09.27.615512. doi: 10.1101/2024.09.27.615512. bioRxiv. 2024. PMID: 39386476 Free PMC article. Preprint. - Zika virus NS3 drives the assembly of a viroplasm-like structure.
Sultana T, Zheng C, Morton G, Megraw TL. Sultana T, et al. bioRxiv [Preprint]. 2024 Sep 16:2024.09.16.613201. doi: 10.1101/2024.09.16.613201. bioRxiv. 2024. PMID: 39345390 Free PMC article. Preprint. - Distinct Replication Kinetics, Cytopathogenicity, and Immune Gene Regulation in Human Microglia Cells Infected with Asian and African Lineages of Zika Virus.
Bird IM, Cavener V, Surendran Nair M, Nissly RH, Chothe SK, Jacob J, Kuchipudi SV. Bird IM, et al. Microorganisms. 2024 Sep 5;12(9):1840. doi: 10.3390/microorganisms12091840. Microorganisms. 2024. PMID: 39338514 Free PMC article.
References
- Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35:183–193. - PubMed
- Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonca MC, de Oliveira L, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00095-5. - DOI - PubMed
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 NS047344/NS/NINDS NIH HHS/United States
- R21 AI111250/AI/NIAID NIH HHS/United States
- R21 NS095348/NS/NINDS NIH HHS/United States
- R21 AI119530/AI/NIAID NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
- R01 NS047344/NS/NINDS NIH HHS/United States
- R01 MH102690/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical