Seasonally Changing Cryptochrome 1b Expression in the Retinal Ganglion Cells of a Migrating Passerine Bird - PubMed (original) (raw)
Seasonally Changing Cryptochrome 1b Expression in the Retinal Ganglion Cells of a Migrating Passerine Bird
Christine Nießner et al. PLoS One. 2016.
Abstract
Cryptochromes, blue-light absorbing proteins involved in the circadian clock, have been proposed to be the receptor molecules of the avian magnetic compass. In birds, several cryptochromes occur: Cryptochrome 2, Cryptochrome 4 and two splice products of Cryptochrome 1, Cry1a and Cry1b. With an antibody not distinguishing between the two splice products, Cryptochrome 1 had been detected in the retinal ganglion cells of garden warblers during migration. A recent study located Cry1a in the outer segments of UV/V-cones in the retina of domestic chickens and European robins, another migratory species. Here we report the presence of cryptochrome 1b (eCry1b) in retinal ganglion cells and displaced ganglion cells of European Robins, Erithacus rubecula. Immuno-histochemistry at the light microscopic and electron microscopic level showed eCry1b in the cell plasma, free in the cytosol as well as bound to membranes. This is supported by immuno-blotting. However, this applies only to robins in the migratory state. After the end of the migratory phase, the amount of eCry1b was markedly reduced and hardly detectable. In robins, the amount of eCry1b in the retinal ganglion cells varies with season: it appears to be strongly expressed only during the migratory period when the birds show nocturnal migratory restlessness. Since the avian magnetic compass does not seem to be restricted to the migratory phase, this seasonal variation makes a role of eCry1b in magnetoreception rather unlikely. Rather, it could be involved in physiological processes controlling migratory restlessness and thus enabling birds to perform their nocturnal flights.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
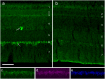
Fig 1. Immuno-labeling for eCry1b in the retina of European Robins.
Retina of a robin (a) in migratory state, and of one (b) after the end of the migratory period. In (a), there is eCry1b labeling in the ganglion cells (layer 6) and the few displaced ganglion cells (layer 4). eCry1b label is located in the cytosol of the cell; the nuclei, indicated by arrows, show no label. In (b), the labeling in the ganglion cells is very low (see also Fig B in S1 Supporting Information). (c-e) Retinal ganglion cell layer in a robin in migratory state, triple-labeled for (c) eCry1b, (d) NeuN, and (e) DAPI. Practically all cells in the ganglion cell layer express eCry1b. Layers of the retina: 1, photoreceptor outer and inner segments; 2, outer nuclear layer; 3, outer plexiform layer; 4, inner nuclear layer; 5, inner plexiform layer; 6, ganglion cell layer; 7, optic nerve fibre layer. The scale bar is 50 μm for all panels.
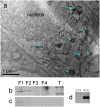
Fig 2. Electron-microscopic image of a ganglion cell, and Western blots.
(a) Ganglion cell in the retina of a robin in migratory state. eCry1b labeling is visualized with diaminobenzidine and silver intensification, visible as dark dots (some marked by arrows). Other subcellular components cannot be identified; eCry1b is probably free in the cytosol and also bound to membranes. (b, c) Western blots of the robin retina, indicating eCry1b (b; ~65 kDa) and eCry1a (c; ~70 kDa) in the cytosolic and in the membrane fraction. Both cryptochromes were detected in the same blot; the part showing eCry1a was already published in [15]. F1, cytosolic fraction; F2, membrane fraction; F3; nuclear fraction; F4, cytoskeletal fraction; T, tongue tissue from the same bird as control. (d) Western blot of purified eCry1a and eCry1b that had been treated with the eCry1b antiserum as control for the specificity of the antiserum, indicating that there is no cross-reactivity of the eCry1b antiserum with eCry1a.
Similar articles
- The Magnetic Compass of Birds: The Role of Cryptochrome.
Wiltschko R, Nießner C, Wiltschko W. Wiltschko R, et al. Front Physiol. 2021 May 19;12:667000. doi: 10.3389/fphys.2021.667000. eCollection 2021. Front Physiol. 2021. PMID: 34093230 Free PMC article. Review. - Double-Cone Localization and Seasonal Expression Pattern Suggest a Role in Magnetoreception for European Robin Cryptochrome 4.
Günther A, Einwich A, Sjulstok E, Feederle R, Bolte P, Koch KW, Solov'yov IA, Mouritsen H. Günther A, et al. Curr Biol. 2018 Jan 22;28(2):211-223.e4. doi: 10.1016/j.cub.2017.12.003. Epub 2018 Jan 4. Curr Biol. 2018. PMID: 29307554 - Localisation of the Putative Magnetoreceptive Protein Cryptochrome 1b in the Retinae of Migratory Birds and Homing Pigeons.
Bolte P, Bleibaum F, Einwich A, Günther A, Liedvogel M, Heyers D, Depping A, Wöhlbrand L, Rabus R, Janssen-Bienhold U, Mouritsen H. Bolte P, et al. PLoS One. 2016 Mar 8;11(3):e0147819. doi: 10.1371/journal.pone.0147819. eCollection 2016. PLoS One. 2016. PMID: 26953791 Free PMC article. - Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass.
Möller A, Sagasser S, Wiltschko W, Schierwater B. Möller A, et al. Naturwissenschaften. 2004 Dec;91(12):585-8. doi: 10.1007/s00114-004-0578-9. Epub 2004 Nov 17. Naturwissenschaften. 2004. PMID: 15551029 - Sensing magnetic directions in birds: radical pair processes involving cryptochrome.
Wiltschko R, Wiltschko W. Wiltschko R, et al. Biosensors (Basel). 2014 Jul 24;4(3):221-42. doi: 10.3390/bios4030221. eCollection 2014 Sep. Biosensors (Basel). 2014. PMID: 25587420 Free PMC article. Review.
Cited by
- Genetic analysis of cryptochrome in insect magnetosensitivity.
Kyriacou CP, Rosato E. Kyriacou CP, et al. Front Physiol. 2022 Aug 10;13:928416. doi: 10.3389/fphys.2022.928416. eCollection 2022. Front Physiol. 2022. PMID: 36035470 Free PMC article. - The magnetic map sense and its use in fine-tuning the migration programme of birds.
Heyers D, Elbers D, Bulte M, Bairlein F, Mouritsen H. Heyers D, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Jul;203(6-7):491-497. doi: 10.1007/s00359-017-1164-x. Epub 2017 Apr 1. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28365788 Review. - The Magnetic Compass of Birds: The Role of Cryptochrome.
Wiltschko R, Nießner C, Wiltschko W. Wiltschko R, et al. Front Physiol. 2021 May 19;12:667000. doi: 10.3389/fphys.2021.667000. eCollection 2021. Front Physiol. 2021. PMID: 34093230 Free PMC article. Review. - Localisation of cryptochrome 2 in the avian retina.
Einwich A, Seth PK, Bartölke R, Bolte P, Feederle R, Dedek K, Mouritsen H. Einwich A, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022 Jan;208(1):69-81. doi: 10.1007/s00359-021-01506-1. Epub 2021 Oct 22. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022. PMID: 34677638 Free PMC article. - Expression patterns of cryptochrome genes in avian retina suggest involvement of Cry4 in light-dependent magnetoreception.
Pinzon-Rodriguez A, Bensch S, Muheim R. Pinzon-Rodriguez A, et al. J R Soc Interface. 2018 Mar;15(140):20180058. doi: 10.1098/rsif.2018.0058. J R Soc Interface. 2018. PMID: 29593090 Free PMC article.
References
- Cashmore AR. Cryptochromes: enabling plants and animals to determine circadian time. Cell. 2003; 114: 537–543. - PubMed
- Haque R, Chaurasia SS, Wessel JH, Iovome PM. Dual regulation of cryptochrome 1 mRNA expression in chicken retina by light and circadian oscillators. NeuroReport. 2002; 13: 2247–2251. - PubMed
- Bailey MJ, Chong NW, Xiong J, Cassone VM. Chickens' Cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors. FEBS Letters. 2002; 513: 160–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
Funded by Deutsche Forschungsgemeinschaft (Grant to RW) and Alfons und Gertrud Kassel Stiftung (Scholarship of CN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources