Localisation of the Putative Magnetoreceptive Protein Cryptochrome 1b in the Retinae of Migratory Birds and Homing Pigeons - PubMed (original) (raw)
Localisation of the Putative Magnetoreceptive Protein Cryptochrome 1b in the Retinae of Migratory Birds and Homing Pigeons
Petra Bolte et al. PLoS One. 2016.
Abstract
Cryptochromes are ubiquitously expressed in various animal tissues including the retina. Some cryptochromes are involved in regulating circadian activity. Cryptochrome proteins have also been suggested to mediate the primary mechanism in light-dependent magnetic compass orientation in birds. Cryptochrome 1b (Cry1b) exhibits a unique carboxy terminus exclusively found in birds so far, which might be indicative for a specialised function. Cryptochrome 1a (Cry1a) is so far the only cryptochrome protein that has been localised to specific cell types within the retina of migratory birds. Here we show that Cry1b, an alternative splice variant of Cry1a, is also expressed in the retina of migratory birds, but it is primarily located in other cell types than Cry1a. This could suggest different functions for the two splice products. Using diagnostic bird-specific antibodies (that allow for a precise discrimination between both proteins), we show that Cry1b protein is found in the retinae of migratory European robins (Erithacus rubecula), migratory Northern Wheatears (Oenanthe oenanthe) and pigeons (Columba livia). In all three species, retinal Cry1b is localised in cell types which have been discussed as potentially well suited locations for magnetoreception: Cry1b is observed in the cytosol of ganglion cells, displaced ganglion cells, and in photoreceptor inner segments. The cytosolic rather than nucleic location of Cry1b in the retina reported here speaks against a circadian clock regulatory function of Cry1b and it allows for the possible involvement of Cry1b in a radical-pair-based magnetoreception mechanism.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
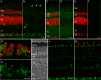
Fig 1. In the pigeon retina, Cry1b was expressed in ganglion cells, putative displaced ganglion cells and photoreceptor inner segments.
Vertical slices of pigeon retina labelled with gwCry1b antibody (A, B green) and Roti® Mount nuclear marker (A, red) showed strong expression of Cry1b protein in the cytoplasm of ganglion cells and single cells in the proximal INL (B, arrow) and weak Cry1b expression in the outer parts of photoreceptors (B, arrowheads). This labelling was absent in controls with pre-immune serum (C, D) and in controls with gwCry1b antibody blocked by the accordant gwCry1b peptides (E, F). Projection (10.1 μm) of gwCry1b labelling (G, H green) together with the Roti® Mount nuclear marker (G red) in the ganglion cell layer showed strong Cry1b expression in the cytoplasm of several ganglion cells, whereas weak or no staining was observed in other ganglion cells (G, H asterisks). With the bright field image for comparison (I), Cry1b immunoreactivity (J, K green) in the photoreceptors was located distal to the outer limiting membrane and proximal to the pigment epithelium. Double immunostaining (K) revealed that Cry1b immunoreactivity (K green) in the outer parts of photoreceptors was not present in outer segments of OPN1SW labelled cones (K red), but in the photoreceptor inner segments proximal to the outer segments. Images (A-F) are maximum projections of confocal stacks and were taken from the same experiment with identical microscope settings and without any image adjustments**.** Scale bars: A-F, 10 μm G, H, 5 μm; I, J, K 10 μm. PE, pigment epithelium; OS, photoreceptor outer segments; IS, photoreceptor inner segments; OLM, outer limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
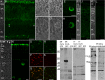
Fig 2. Specificity of gwCry1b antibody.
N2a cells expressing a gwCry1b-GFP fusion protein (A green) stained with gwCry1b antibody (B red) indicated that the antibody detects gwCry1b-GFP protein (C yellow). The same labelling of gwCry1a-GFP expressing cells (D-F) indicated no detection of gwCry1a protein. In Western blots, gwCry1b antibody detected a single protein of the expected size (~64 kDa) in samples of purified gwCry1b protein (G left lane) but not in samples of gwCry1a protein (G right lane). Western blots incubated with gwCry1b antibody showed a band of ~64 kDa on retinal total homogenates from pigeons (H left lane) and purified gwCry1b protein (H right lane). No bands were detected in blots incubated with pre-immune serum (I) and gwCry1b antibody blocked by gwCry1b peptides (J). In G-J, the molecular mass in kDa is indicated on the left. All images (A-E) are maximum projections of confocal stacks and were taken from the same experiment with identical microscope settings and without any image adjustments**.**
Fig 3. Antibodies against erCry1b confirm Cry1b expression of ganglion cells and inner segments in European robin and Northern wheatear.
Immunolabelling of the erCry1b antibody in the retina of a European robin (A) revealed Cry1b expression (B) in several ganglion cells, in the photoreceptor inner segments and in few cells in the INL (B asterisks). Immunoreactive cells in the very proximal INL (C-H) were significantly bigger than surrounding bipolar cells (C, D, F, G) and showed a large cytosolic space. Immunoreactivity pattern shown in B was absent in controls with the erCry1b antibody blocked by erCry1b peptides (J) and in controls using pre-immune serum (L). In the retina of the Northern wheatear (M, N), the erCry1b antibody showed the same pattern as seen in European robins (N). Staining beneath the photoreceptor oil droplets (M arrowheads) and distal to the OLM indicated Cry1b expression in photoreceptor inner segments (M). Immunocytochemistry of N2a cells expressing erCry1b-GFP fusion protein (O green) showed that the erCry1b antibody (P red) detects erCry1b protein (Q yellow in the overlay). Immunolabelling of erCry1a-GFP expressing cells (R green) indicated that the same antibody did not detect Cry1a protein (S, T). In Western blots, the erCry1b antibody detected a band of the expected size of ~92 kDa in protein homogenates of erCry1b-GFP expressing N2a cells (U middle lane). No band at the appropriate molecular mass was seen in homogenates of erCry1a-GFP (U left lane) or GFP expressing cells (U right lane). The signal at the appropriate molecular mass was absent in negative controls after probing the blot with pre-immune serum (V). Western blots on protein homogenates from retinae of European robins incubated with the erCry1b antibody showed a band of the appropriate size ~64 kDa (W asterisk). This band was absent in controls when the blot was incubated with the pre-adsorbed antibody (Q). Molecular mass marker proteins are indicated on the left (kDa). All confocal images are maximum projections (B, J, L 12.8 μm; N, 3 μm). Images A-L were taken with identical settings. PE, pigment epithelium; IS, photoreceptor inner segments; OLM, outer limiting membrane; ONL outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars: A, B 50 μm; D-H, M, N 10 μm; I-L, O-T 25 μm.
Similar articles
- Double-Cone Localization and Seasonal Expression Pattern Suggest a Role in Magnetoreception for European Robin Cryptochrome 4.
Günther A, Einwich A, Sjulstok E, Feederle R, Bolte P, Koch KW, Solov'yov IA, Mouritsen H. Günther A, et al. Curr Biol. 2018 Jan 22;28(2):211-223.e4. doi: 10.1016/j.cub.2017.12.003. Epub 2018 Jan 4. Curr Biol. 2018. PMID: 29307554 - Seasonally Changing Cryptochrome 1b Expression in the Retinal Ganglion Cells of a Migrating Passerine Bird.
Nießner C, Gross JC, Denzau S, Peichl L, Fleissner G, Wiltschko W, Wiltschko R. Nießner C, et al. PLoS One. 2016 Mar 8;11(3):e0150377. doi: 10.1371/journal.pone.0150377. eCollection 2016. PLoS One. 2016. PMID: 26953690 Free PMC article. - Localisation of cryptochrome 2 in the avian retina.
Einwich A, Seth PK, Bartölke R, Bolte P, Feederle R, Dedek K, Mouritsen H. Einwich A, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022 Jan;208(1):69-81. doi: 10.1007/s00359-021-01506-1. Epub 2021 Oct 22. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2022. PMID: 34677638 Free PMC article. - The Radical-Pair Mechanism of Magnetoreception.
Hore PJ, Mouritsen H. Hore PJ, et al. Annu Rev Biophys. 2016 Jul 5;45:299-344. doi: 10.1146/annurev-biophys-032116-094545. Epub 2016 May 16. Annu Rev Biophys. 2016. PMID: 27216936 Review. - The Magnetic Compass of Birds: The Role of Cryptochrome.
Wiltschko R, Nießner C, Wiltschko W. Wiltschko R, et al. Front Physiol. 2021 May 19;12:667000. doi: 10.3389/fphys.2021.667000. eCollection 2021. Front Physiol. 2021. PMID: 34093230 Free PMC article. Review.
Cited by
- Weak Broadband Electromagnetic Fields are More Disruptive to Magnetic Compass Orientation in a Night-Migratory Songbird (Erithacus rubecula) than Strong Narrow-Band Fields.
Schwarze S, Schneider NL, Reichl T, Dreyer D, Lefeldt N, Engels S, Baker N, Hore PJ, Mouritsen H. Schwarze S, et al. Front Behav Neurosci. 2016 Mar 22;10:55. doi: 10.3389/fnbeh.2016.00055. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27047356 Free PMC article. - A novel isoform of cryptochrome 4 (Cry4b) is expressed in the retina of a night-migratory songbird.
Einwich A, Dedek K, Seth PK, Laubinger S, Mouritsen H. Einwich A, et al. Sci Rep. 2020 Sep 25;10(1):15794. doi: 10.1038/s41598-020-72579-2. Sci Rep. 2020. PMID: 32978454 Free PMC article. - Inhomogeneous ensembles of radical pairs in chemical compasses.
Procopio M, Ritz T. Procopio M, et al. Sci Rep. 2016 Nov 2;6:35443. doi: 10.1038/srep35443. Sci Rep. 2016. PMID: 27804956 Free PMC article. - Magnetic activation in the brain of the migratory northern wheatear (Oenanthe oenanthe).
Elbers D, Bulte M, Bairlein F, Mouritsen H, Heyers D. Elbers D, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017 Aug;203(8):591-600. doi: 10.1007/s00359-017-1167-7. Epub 2017 Mar 30. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017. PMID: 28361169 - The expression, localisation and interactome of pigeon CRY2.
Balay SD, Hochstoeger T, Vilceanu A, Malkemper EP, Snider W, Dürnberger G, Mechtler K, Schuechner S, Ogris E, Nordmann GC, Ushakova L, Nimpf S, Keays DA. Balay SD, et al. Sci Rep. 2021 Oct 13;11(1):20293. doi: 10.1038/s41598-021-99207-x. Sci Rep. 2021. PMID: 34645873 Free PMC article.
References
- Wiltschko W, Wiltschko R (1972) Magnetic compass of European robins. Science 176: 62–64. - PubMed
- Wiltschko W, Wiltschko R (1995) Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J Comp Physiol 177: 363–369.
- Cochran WW, Mouritsen H, Wikelski M (2004) Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304: 405–408. - PubMed
- Kishkinev DA, Chernetsov NS (2015) Magnetoreception systems in birds: A review of current research. Biol Bull Rev 5: 46–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
Study design, data collection and analysis was supported and funded by a Lichtenberg Professorship from the VolkswagenStiftung to HM, by the Niedersaechsisches Ministerium für Wissenschaft und Kultur to HM, by the Deutsche Forschungsgemeinschaft (GRK 1885: molecular mechanisms of sensory biology) to UJB, RR, AE and HM, by Defense Adavanced Research Projects Agency (QuBE_ N66001-10-1-4061) to HM, by the Deutsche Forschungsgemeinschaft (HE6221/1-1) to DH, and MO 1408/1-2 to HM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources