The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway - PubMed (original) (raw)
. 2016 May 15;310(10):E828-37.
doi: 10.1152/ajpendo.00056.2016. Epub 2016 Mar 8.
Yi Yang 2, Yun Zhong 3, Hala Mustafa Ammar 4, Peihua Zhang 1, Runmin Guo 1, Hua Liu 2, Chuanfang Cheng 3, Thomas M Koroscil 5, Yanfang Chen 6, Shiming Liu 3, Ji C Bihl 7
Affiliations
- PMID: 26956185
- PMCID: PMC4895450
- DOI: 10.1152/ajpendo.00056.2016
The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway
Keng Wu et al. Am J Physiol Endocrinol Metab. 2016.
Abstract
Our previous study showed that circulating microvesicles (cMVs) of diabetic mice have negative effects on the function of endothelial progenitor cells (EPCs). Whether this is true in diabetic patients deserves further study. In this study, the effects of cMVs and EPC-derived MVs (EPC-MVs) on EPC migration, apoptosis, and reactive oxygen species (ROS) production in healthy controls, well-controlled, and uncontrolled diabetic patients were investigated. The levels of miR-126 and vascular endothelial growth factor receptor 2 (VEGFR2) in cMVs, EPC-MVs, and/or EPCs were analyzed. Moreover, miR-126 inhibitor or mimic was applied to EPCs to modulate the miR-126 level in EPC-MVs. We found the following: 1) the circulating EPC level was reduced but the circulating EPC-MV level increased in uncontrolled diabetic patients; 2) the cMVs and EPC-MVs of healthy controls had beneficial effects on EPCs (migration, apoptosis, ROS), whereas the effects were reversely changed in the cMVs and EPC-MVs of uncontrolled diabetic patients; and 3) the cMVs and EPC-MVs of uncontrolled diabetic patients carried less miR-126 and had downregulated VEGFR2 expression in EPCs. Manipulating the miR-126 level in EPC-MVs with inhibitor or mimic changed their function. The effects of cMVs and EPC-MVs are compromised in diabetes due to the reduction of their carried miR-126, which might provide a therapy target for diabetic vascular complications.
Keywords: endothelial progenitor cells; miR-126; microvesicles; oxidative stress; type 2 diabetes; vascular endothelial growth factor receptor 2.
Copyright © 2016 the American Physiological Society.
Figures
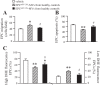
Fig. 1.
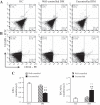
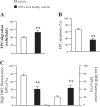
The levels of circulating endothelial progenitor cells (EPCs) and EPC-derived microvesicles (EPC-MVs). A and B: representative flow cytometry plots of circulating EPCs (A) and EPC-MVs (B) in healthy controls (HC) and diabetes mellitus (DM) patients. C: summarized data on circulating EPC and EPC-MV levels; n = 21 for HC, 14 for well-controlled DM, and 10 for uncontrolled DM. **P < 0.01 vs. HC; ++P < 0.01 vs. well-controlled DM by 2-way ANOVA. VEGFR, VEGF receptor; PE, phycoerythrin.
Fig. 2.
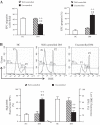
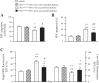
Migration ability, apoptotic rate, and reactive oxygen species (ROS) production of EPCs from diabetic patients and HC. A: summarized data on migration ability and apoptotic rate. B: representative flow cytometry plots and summarized data of ROS production. P1, EPCs with low ROS; P2, EPCs with high ROS; n = 21 for HC, 14 for well-controlled DM, and 10 for uncontrolled DM. **P < 0.01 vs. HC; ++P < 0.01 vs. well-controlled DM by 2-way ANOVA. DHE, dihydroethidium.
Fig. 3.
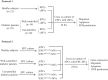
Diagram depicts the design of cross-coculture experiments for determining the effects of circulating MVs (cMVs) and EPC-MVs on EPCs. In protocol 1, EPCs from HC were cocultured with cMVs from well-controlled or uncontrolled diabetic patients, whereas EPCs from uncontrolled diabetic patients were cocultured with cMVs from HC. In protocol 2, EPCs from healthy controls were cocultured with EPC-MVs released from EPCs of diabetic patients transfected with miR-126c or miR-126m, whereas EPCs from uncontrolled diabetic patients were cocultured with EPC-MVs released from EPCs of healthy controls transfected with miR-126c or miR-126i. After coculture, the expressions of miR-126 and VEGFR2 and migration, apoptosis, and ROS production of EPCs were measured. EPCmiR-126c-MVs, EPCmiR-126i-MVs, and EPCmiR-126m-MVs, microvesicles released from EPCs transfected with miR-126 control, inhibitor, and mimic, respectively.
Fig. 4.
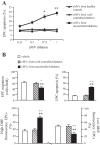
Coculture study of cMVs from uncontrolled diabetic patients with EPCs from healthy controls. A: dose-dependent effect of cMVs from HC and diabetic patients on heathy EPC apoptotic rate; n = 3. *P < 0.05 and **P < 0.01 vs. 0.25. B: effects of cMVs from diabetic patients on healthy EPC migration, apoptotic rate, and ROS production; n = 6. **P < 0.01 vs. vehicle, +P < 0.05 and ++P < 0.01 vs. cMVs from well-controlled diabetic patients.
Fig. 5.
Coculture study of cMVs from HC with EPCs from uncontrolled diabetic patients. A: effects of cMVs from HC on the migration of EPCs from uncontrolled diabetic patients. B: effects of cMVs from HC on the apoptotic rate of EPCs from uncontrolled diabetic patients. C: the effects of cMVs from HC on the ROS production of EPCs from uncontrolled diabetic patients; n = 6. **P < 0.01 vs. vehicle.
Fig. 6.
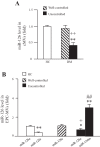
The expression of miR-126 in cMVs and EPC-MVs. A: expression of miR-126 in cMVs isolated HC and well-controlled or uncontrolled DM. B: miR-126 expression in EPC-MVs released from EPCs of HC and well-controlled or uncontrolled DM. EPCs were transfected with miR-126 control, mimic, or inhibitor; n = 6. *P < 0.05 and **P < 0.01 vs. cMVs or EPC-MVs from healthy controls; +P < 0.05 and ++P <0.01 vs. cMVs or EPC-MVs from well-controlled diabetes; #P < 0.05 and ##P < 0.01 vs. cMVs or EPC-MVs from uncontrolled diabetes.
Fig. 7.
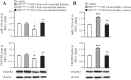
Expressions of miR-126 and VEGFR2 in EPCs after coincubation with EPC-MVs. A: expressions of miR-126 and VEGFR2 in EPCs from HC after coculture with EPC-MVs released from EPCs of well-controlled or uncontrolled diabetes. Data are expressed as a fold change of vehicle; n = 6. *P < 0.05 vs. vehicle; +P < 0.05 vs. EPCmiR-126c-MVs from well-controlled diabetes; ##P < 0.01 vs. EPCmiR-126c-MVs from uncontrolled diabetes. B: EPC-MVs released from EPCs of HC increase the expression of miR-126 and VEGFR2 in EPCs from uncontrolled diabetes, which can be reversed by miR-126i transfection. Data are expressed as a fold change of vehicle; n = 6. **P < 0.01 vs. vehicle; ##P < 0.01 vs. EPCmiR-126c-MVs from HC.
Fig. 8.
Effects of EPC-MVs released from EPCs of uncontrolled diabetes on the EPCs from HC. A: the effects of EPC-MVs released from EPCs of uncontrolled diabetes on the migration of EPCs from HC. B: effects of EPC-MVs released from EPCs of uncontrolled diabetes on the apoptotic rate of EPCs from HC. C: effects of EPC-MVs released from EPCs of uncontrolled diabetes on the ROS production of EPCs from HC; n = 6. *P < 0.05 and **P < 0.01 vs. vehicle; +P < 0.05 and ++P < 0.01 vs. EPCmiR-126c-MVs from well-controlled diabetes; #P < 0.05 and ##P < 0.01 vs. EPCmiR-126c-MVs from uncontrolled diabetes.
Fig. 9.
Effects of EPC-MVs released from EPCs of HC on the EPCs from uncontrolled diabetes. A: effects of EPC-MVs released from EPCs of HC on the migration of EPCs from uncontrolled diabetes. B: effects of EPC-MVs released from EPCs of HC on the apoptotic rate of EPCs from uncontrolled diabetes. C: effects of EPC-MVs released from EPCs of HC on the ROS production of EPCs from uncontrolled diabetes; n = 6. *P <0.05 and **P <0.01 vs. vehicle; #P <0.05 vs. EPCmiR-126c-MVs from HC.
Similar articles
- Enrichment of miR-126 enhances the effects of endothelial progenitor cell-derived microvesicles on modulating MC3T3-E1 cell function via Erk1/2-Bcl-2 signalling pathway.
Chen G, Li P, Liu Z, Zeng R, Ma X, Chen Y, Xu H, Li Z, Lin H. Chen G, et al. Prion. 2019 Jan;13(1):106-115. doi: 10.1080/19336896.2019.1607464. Prion. 2019. PMID: 31050590 Free PMC article. - Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1.
Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Meng S, et al. J Mol Cell Cardiol. 2012 Jul;53(1):64-72. doi: 10.1016/j.yjmcc.2012.04.003. Epub 2012 Apr 16. J Mol Cell Cardiol. 2012. PMID: 22525256 - Multiple therapeutic effect of endothelial progenitor cell regulated by drugs in diabetes and diabetes related disorder.
Ambasta RK, Kohli H, Kumar P. Ambasta RK, et al. J Transl Med. 2017 Aug 31;15(1):185. doi: 10.1186/s12967-017-1280-y. J Transl Med. 2017. PMID: 28859673 Free PMC article. Review. - Pathogenic roles of microvesicles in diabetic retinopathy.
Zhang W, Chen S, Liu ML. Zhang W, et al. Acta Pharmacol Sin. 2018 Jan;39(1):1-11. doi: 10.1038/aps.2017.77. Epub 2017 Jul 17. Acta Pharmacol Sin. 2018. PMID: 28713160 Free PMC article. Review.
Cited by
- Type 2 diabetes and susceptibility to atrial fibrillation: the two facets of downregulation of MiR-126?
Mormile R. Mormile R. Cardiovasc Endocrinol Metab. 2018 Aug 15;7(3):68-69. doi: 10.1097/XCE.0000000000000156. eCollection 2018 Sep. Cardiovasc Endocrinol Metab. 2018. PMID: 31646285 Free PMC article. No abstract available. - MicroRNA-126 enhances the biological function of endothelial progenitor cells under oxidative stress via PI3K/Akt/GSK3β and ERK1/2 signaling pathways.
Wu Q, Qi B, Duan X, Ming X, Yan F, He Y, Bu X, Sun S, Zhu H. Wu Q, et al. Bosn J Basic Med Sci. 2021 Feb 1;21(1):71-80. doi: 10.17305/bjbms.2019.4493. Bosn J Basic Med Sci. 2021. PMID: 31999938 Free PMC article. - The Role of MicroRNAs in Diabetes-Related Oxidative Stress.
Qadir MMF, Klein D, Álvarez-Cubela S, Domínguez-Bendala J, Pastori RL. Qadir MMF, et al. Int J Mol Sci. 2019 Oct 31;20(21):5423. doi: 10.3390/ijms20215423. Int J Mol Sci. 2019. PMID: 31683538 Free PMC article. Review. - Compromised endothelial progenitor cell exosomal communication with endothelial cells in hypertension ischemia conditions.
Chen S, Polaki V, Bihl JC, Wang J. Chen S, et al. Front Stroke. 2022;1:1015463. doi: 10.3389/fstro.2022.1015463. Epub 2022 Oct 13. Front Stroke. 2022. PMID: 39450345 Free PMC article. - Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart.
Walkowski B, Kleibert M, Majka M, Wojciechowska M. Walkowski B, et al. Cells. 2022 May 5;11(9):1553. doi: 10.3390/cells11091553. Cells. 2022. PMID: 35563860 Free PMC article. Review.
References
- Burger D, Montezano AC, Nishigaki N, He Y, Carter A, Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 31: 1898–1907, 2011. - PubMed
- Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens 6: 85–99, 2012. - PubMed
- Cai HY, Li L, Guo T, Wang YU, Ma TK, Xiao JM, Zhao L, Fang Y, Yang P, Zhao HU. Cardiac shockwave therapy improves myocardial function in patients with refractory coronary artery disease by promoting VEGF and IL-8 secretion to mediate the proliferation of endothelial progenitor cells. Exp Ther Med 10: 2410–2416, 2015. - PMC - PubMed
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical