Integrin-linked kinase activity modulates the pro-metastatic behavior of ovarian cancer cells - PubMed (original) (raw)
Integrin-linked kinase activity modulates the pro-metastatic behavior of ovarian cancer cells
Lana Bruney et al. Oncotarget. 2016.
Abstract
Epithelial ovarian cancer (EOC) is the most fatal gynecologic cancer in the U.S., resulting in >14,000 deaths/year. Most women are diagnosed at late stage with widely disseminated intra-peritoneal metastatic disease, resulting in a 5-year survival rate of <30%. EOCs spread via direct extension and exfoliation into the peritoneal cavity, adhesion to peritoneal mesothelial cells, mesothelial cell retraction to expose sub-mseothelial matrix and anchoring in the type I collagen-rich matrix to generate secondary lesions. As a molecular-level understanding of EOC metastasis may identify novel therapeutic targets, the current study evaluated the expression and activity of integrin-linked kinase (ILK), a Ser/Thr protein kinase activated upon integrin-mediated adhesion. Results show that ILK is co-expressed in EOC with the pro-metastatic enzyme membrane type 1 matrix metalloproteinase (MT1-MMP) and catalyzed phosphorylation of the cytoplasmic tail of the proteinase. Downregulation of ILK expression or activity reduced adhesion to and invasion of collagen gels and organotypic meso-mimetic cultures. As an initial early event in EOC metastasis is integrin-mediated adhesion, these results suggest that further evaluation of ILK inhibitors as anti-metastatic agents in EOC is warranted.
Keywords: integrin; integrin linked kinase; membrane type 1 matrix metalloproteinase; metastasis; ovarian cancer.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures
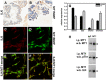
Figure 1. Expression of ILK and MT1-MMP in human ovarian carcinoma tissues and cells
A, B. Microarrayed cores of ovarian adenocarcinoma were subjected to immunohistochemical analyses for ILK (A) or MT1-MMP (B) as described in Materials and Methods. 20x magnification. C-F. Fluorescence co-localization of ILK and MT1-MMP in DOV13 human ovarian cancer cells. Subconfluent monolayers of cells were seeded onto collagen type I coated coverslips and processed for immunofluorescence. (C) ILK (red), (D) MT1-MMP (green), (E, F) representative merged images. G. Quantitative real time PCR analysis of ILK and MT1-MMP in ovarian cancer cells. The comparative CT method was used to determine average relative quantitation. Results represent the mean of a minimum of three independent experiments. H. Immunoprecipitation of MT1-MMP. Cell lysates were subjected to immunoprecipitation with anti-MT1-MMP antibody (designated ‘MT1’) or control IgG and protein G beads. Immunoprecipitates were electrophoresed on 9% polyacrylamide gels and subject to western blotting using anti-MT1-MMP (upper panel), anti-phospho-T_X_R antibodies (middle panel) or anti-ILK antibodies (lower panel), followed by secondary antibodies and enhanced chemiluminescent detection.
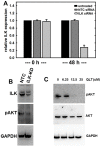
Figure 2. Regulation of ILK expression and activity
A. siRNA-mediated silencing of ILK expression as confirmed using quantitative real time PCR analysis of ILK levels as described in Materials and Methods. Results are expressed relative to non-targeting control siRNA (NTC) and represent the mean of a minimum of three independent experiments. A Mann-Whitney U test was employed to determine statistical significance between NTC siRNA and ILK-siRNA as indicated. No difference between untreated and NTC siRNA was detected at any time point (0 h, p=0.479; 48 h, p=0.487). A significant difference is observed comparing either untreated or NTC-siRNA with ILK-siRNA at 48 h (p<.001 in both cases). B. Western blot analysis showing expression of ILK (upper panel) in cells transfected with NTC-siRNA or ILK-siRNA as indicated. In the middle panel, cell lysate was probed with the ILK downstream mediator phospho-AKT-Ser473. Lower panel is probed with GAPDH as a loading control. C. Western blot analysis showing inhibition of ILK activity using QLT0267, as determined by evaluating phosphorylation of AKT. Cells were treated with the ILK inhibitor QLT0267 or vehicle control, as indicated. Cell lysates were probed with antibodies directed against phospho-AKT-Ser473 (upper panel), total AKT (middle panel), or GAPDH (lower panel) as indicated.
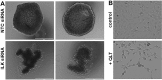
Figure 3. Reduced ILK modulates MCA formation and cell spreading
A. MCAs were generated using the hanging drop method as described in Materials and Methods. Representative examples of two MCAs formed from NTC-siRNA control cells (control, upper panels) and ILK-knockdown cells (ILK-siRNA, lower panels) are shown. Scale bar, 400 um. B. Cells were plated on coverslips and incubated with vehicle (control, upper panel) or the ILK inhibitor QLT0267 (+QLT, lower panel, 25 uM) for 12 h prior to visualization with light microscopy.
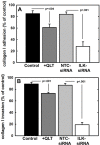
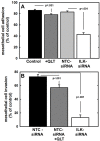
Figure 4. Effect of modified ILK expression and activity on adhesion to and invasion of type I collagen
A. Adhesion to type I collagen. Untreated control cells (control, black bar), cells treated with QLT0267 (25 uM, dark grey bar), cells transfected with non-targeting control siRNA (NTC-siRNA, light grey bar) or cells transfected with ILK-targeting siRNA (ILK-siRNA, white bar) were incubated in wells coated with type I collagen for 30 min prior to washing to remove unbound cells and quantitation of adherent cells. Results are depicted as the percentage of total cells seeded allowed to adhere overnight and represent the mean of three independent experiments. Statistical significance was determined using Mann-Whitney U tests and p values are indicated. B. Invasion of 3-dimensional collagen gels. Cells, defined as in (A), were seeded into transwells containing a 8 um pore filter overlaid with a 3-dimensional collagen gel as described in Materials and Methods. After incubation for 24 h, non-invading cells were scraped from the top of the filter, the filter was removed and stained. Invading cells adherent to the underside of the filter were enumerated. Results represent the percentage of cells migrated relative to total cells seeded and represent the mean of three independent experiments. Statistical significance was determined using Mann-Whitney U tests and p values are indicated.
Figure 5. Effect of modified ILK expression and activity on adhesion to and invasion of live mesothelial cell monolayers or meso-mimetic cultures
A. Adhesion to mesothelial monolayers. Untreated control cells (control, black bar), cells treated with QLT0267 (25 uM, dark grey bar), cells transfected with non-targeting control siRNA (NTC-siRNA, light grey bar) or cells transfected with ILK-targeting siRNA (ILK-siRNA, white bar) were fluorescently labeled with CMFDA and incubated in wells containing a confluent monolayer of LP9 human peritoneal mesothelial cells for 30 min prior to washing to remove unbound cells and quantitation of adherent cells. Results are depicted as the percentage of total cells seeded allowed to adhere overnight and represent the mean of three independent experiments. Statistical significance was determined using Mann-Whitney U tests and p values are indicated. B. Invasion of meso-mimetic cultures. Control cells transfected with non-targeting siRNA (NTC-siRNA, black bar), NTC-siRNA control cells treated with QLT0267 (NTC-siRNA+QLT, dark grey bar), or cells transfected with ILK-targeting siRNA (ILK-siRNA, white bar) were fluorescently labeled with CMFDA and incubated in transwells containing organotypic meso-mimetic cultures comprised of a confluent layer of LP9 human peritoneal mesothelial cells cultured atop a 3-dimensional type I collagen gel on a porous membrane [57-58]. After incubation for 24 h, cells were scraped from the top of the filter, the filter was removed and stained. Invading cells adherent to the underside of the filter were enumerated. Results represent the percentage of cells migrated relative to total cells seeded and represent the mean of three independent experiments. A Kruskal-Wallis test was used to find a significant mean difference among all three groups (p=0.007). Statistical significance between 2 groups was determined using Mann-Whitney U tests and p values are indicated.
Similar articles
- Cadherin composition and multicellular aggregate invasion in organotypic models of epithelial ovarian cancer intraperitoneal metastasis.
Klymenko Y, Kim O, Loughran E, Yang J, Lombard R, Alber M, Stack MS. Klymenko Y, et al. Oncogene. 2017 Oct 19;36(42):5840-5851. doi: 10.1038/onc.2017.171. Epub 2017 Jun 19. Oncogene. 2017. PMID: 28628116 Free PMC article. - Membrane-type I matrix metalloproteinase-dependent ectodomain shedding of mucin16/ CA-125 on ovarian cancer cells modulates adhesion and invasion of peritoneal mesothelium.
Bruney L, Conley KC, Moss NM, Liu Y, Stack MS. Bruney L, et al. Biol Chem. 2014 Oct;395(10):1221-31. doi: 10.1515/hsz-2014-0155. Biol Chem. 2014. PMID: 25205731 Free PMC article. - Ovarian cancer cell detachment and multicellular aggregate formation are regulated by membrane type 1 matrix metalloproteinase: a potential role in I.p. metastatic dissemination.
Moss NM, Barbolina MV, Liu Y, Sun L, Munshi HG, Stack MS. Moss NM, et al. Cancer Res. 2009 Sep 1;69(17):7121-9. doi: 10.1158/0008-5472.CAN-08-4151. Epub 2009 Aug 25. Cancer Res. 2009. PMID: 19706774 Free PMC article. - Guidance of Signaling Activations by Cadherins and Integrins in Epithelial Ovarian Cancer Cells.
Roggiani F, Mezzanzanica D, Rea K, Tomassetti A. Roggiani F, et al. Int J Mol Sci. 2016 Aug 23;17(9):1387. doi: 10.3390/ijms17091387. Int J Mol Sci. 2016. PMID: 27563880 Free PMC article. Review. - Molecular and cellular mechanisms controlling integrin-mediated cell adhesion and tumor progression in ovarian cancer metastasis: a review.
Dhaliwal D, Shepherd TG. Dhaliwal D, et al. Clin Exp Metastasis. 2022 Apr;39(2):291-301. doi: 10.1007/s10585-021-10136-5. Epub 2021 Nov 25. Clin Exp Metastasis. 2022. PMID: 34822024 Free PMC article. Review.
Cited by
- Integrin-linked kinase-frizzled 7 interaction maintains cancer stem cells to drive platinum resistance in ovarian cancer.
Atwani R, Nagare RP, Rogers A, Prasad M, Lazar V, Sandusky G, Tong Y, Pin F, Condello S. Atwani R, et al. J Exp Clin Cancer Res. 2024 Jun 1;43(1):156. doi: 10.1186/s13046-024-03083-y. J Exp Clin Cancer Res. 2024. PMID: 38822429 Free PMC article. - Gender- and Age-Based Characterization and Comparison of the Murine Primary Peritoneal Mesothelial Cell Proteome.
Wang Z, Liu Y, Safavisohi R, Asem M, Hu DD, Stack MS, Champion MM. Wang Z, et al. bioRxiv [Preprint]. 2024 Oct 11:2024.10.09.617441. doi: 10.1101/2024.10.09.617441. bioRxiv. 2024. PMID: 39416176 Free PMC article. Preprint. - LncRNA MEG3 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN.
Wang J, Xu W, He Y, Xia Q, Liu S. Wang J, et al. Inflamm Res. 2018 Dec;67(11-12):927-936. doi: 10.1007/s00011-018-1186-z. Epub 2018 Oct 11. Inflamm Res. 2018. PMID: 30310931 - Emodin suppresses proliferation, migration and invasion in ovarian cancer cells by down regulating ILK in vitro and in vivo.
Lu J, Xu Y, Zhao Z, Ke X, Wei X, Kang J, Zong X, Mao H, Liu P. Lu J, et al. Onco Targets Ther. 2017 Jul 19;10:3579-3589. doi: 10.2147/OTT.S138217. eCollection 2017. Onco Targets Ther. 2017. PMID: 28790850 Free PMC article. Retracted. - Post-translational modification of the membrane type 1 matrix metalloproteinase (MT1-MMP) cytoplasmic tail impacts ovarian cancer multicellular aggregate dynamics.
Yang J, Kasberg WC, Celo A, Liang Z, Quispe K, Stack MS. Yang J, et al. J Biol Chem. 2017 Aug 11;292(32):13111-13121. doi: 10.1074/jbc.M117.800904. Epub 2017 Jun 27. J Biol Chem. 2017. PMID: 28655772 Free PMC article.
References
- Niedbala MJ, Crickard K, Bernacki RJ. Interactions of human ovarian tumor cells with human mesothelial cells grown on extracellular matrix. An in vitro model system for studying tumor cell adhesion and invasion. Exp Cell Res. 1985;160:499–513. - PubMed
- Ellerbroek SM, Fishman DA, Kearns AS, Bafetti LM, Stack MS. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through beta1 integrin. Cancer Res. 1999;59:1635–1641. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials