The Nuclear Pore Complex as a Flexible and Dynamic Gate - PubMed (original) (raw)
Review
The Nuclear Pore Complex as a Flexible and Dynamic Gate
Kevin E Knockenhauer et al. Cell. 2016.
Abstract
Nuclear pore complexes (NPCs) perforate the nuclear envelope and serve as the primary transport gates for molecular exchange between nucleus and cytoplasm. Stripping the megadalton complex down to its most essential organizational elements, one can divide the NPC into scaffold components and the disordered elements attached to them that generate a selective barrier between compartments. These structural elements exhibit flexibility, which may hold a clue in understanding NPC assembly and function. Here we review the current status of NPC research with a focus on the functional implications of its structural and compositional heterogeneity.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
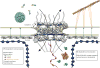
Figure 1. Function of the nuclear pore complex in cellular communication
The nuclear pore (NPC) (human proteins, grey, EMDB code 3103, (Appen et al., 2015) coordinates a multitude of transport processes, including nuclear import and export, and viral interactions. Depicted in the cartoon, human hepatitis B virus (cyan, EMDB code 3015) and an import complex comprising importin-β, importin-α, and cargo (blue, green, and yellow, composite of PDB codes 1QGK, 1EE5, 1K5J, respectively) are ready to traverse the NPC. Exiting the nucleus are the 60S pre-ribosomal subunit (yellow-orange, PDB code 1JJ2, and an mRNP comprising the export factor TAP bound to RNA (pink and purple, PDB code 3RW6), and a protein export complex comprising exportin Cse1-Kap60 Cargo-RanGTP (green, PDB code 1WA5).
Figure 2. Overall structure of the NPC
Cryo-electron tomographic (cryo-ET) reconstruction of the human NPC from HeLa cells (EMDB code 3103, (Appen et al., 2015)). Cut-away view showing half of an NPC embedded in the nuclear envelope (yellow). Additional structural elements, i.e., cytoplasmic filaments and nuclear basket that have been excluded from the cryo-ET analysis, are added schematically. Nucleoporins from yeast and metazoa are listed and color-matched according to their approximate positions within the NPC.
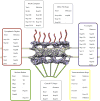
Figure 3. Domain architecture of nucleoporins
Yeast nucleoporins with their metazoan homologs are listed. The domain architecture is derived primarily from X-ray crystallographic data, combined with structure prediction whenever experimental data is not yet available. The vast majority of nucleoporins are built from a limited set of structural modules, which characteristically occur in multiple proteins.
Figure 4. Representative large structures and protein complexes from the NPC
(A) Composite structure built from six overlapping individual crystal structures (Kelley et al., 2015). (B) The topology of the trimeric Nsp1-Nup57-Nup49 complex from fungi is identical to the metazoan Nup62-Nup54-Nup58 complex (Chug et al., 2015; Stuwe et al., 2015a). Both complexes are anchored to the NPC scaffold via Nic96/Nup93. Despite low sequence similarity scaffold structures are largely conserved between evolutionarily highly diverged eukaryotic species. (C) Structure of the large scaffold Nup188 built from three segments: bluegreen and blue elements are crystal structures (Andersen et al., 2013) while the grey segment is modeled. All panels are on the same scale.
Similar articles
- The structure of the nuclear pore complex.
Hoelz A, Debler EW, Blobel G. Hoelz A, et al. Annu Rev Biochem. 2011;80:613-43. doi: 10.1146/annurev-biochem-060109-151030. Annu Rev Biochem. 2011. PMID: 21495847 Review. - Linker Nups connect the nuclear pore complex inner ring with the outer ring and transport channel.
Fischer J, Teimer R, Amlacher S, Kunze R, Hurt E. Fischer J, et al. Nat Struct Mol Biol. 2015 Oct;22(10):774-81. doi: 10.1038/nsmb.3084. Epub 2015 Sep 7. Nat Struct Mol Biol. 2015. PMID: 26344569 - The nuclear pore complex: a strategic platform for regulating cell signaling.
Gu Y. Gu Y. New Phytol. 2018 Jul;219(1):25-30. doi: 10.1111/nph.14756. Epub 2017 Aug 31. New Phytol. 2018. PMID: 28858378 Review. - Structure and gating of the nuclear pore complex.
Eibauer M, Pellanda M, Turgay Y, Dubrovsky A, Wild A, Medalia O. Eibauer M, et al. Nat Commun. 2015 Jun 26;6:7532. doi: 10.1038/ncomms8532. Nat Commun. 2015. PMID: 26112706 Free PMC article. - Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease.
Jamali T, Jamali Y, Mehrbod M, Mofrad MR. Jamali T, et al. Int Rev Cell Mol Biol. 2011;287:233-86. doi: 10.1016/B978-0-12-386043-9.00006-2. Int Rev Cell Mol Biol. 2011. PMID: 21414590 Review.
Cited by
- Identification of the Karyopherin Superfamily in Maize and Its Functional Cues in Plant Development.
Jin L, Zhang G, Yang G, Dong J. Jin L, et al. Int J Mol Sci. 2022 Nov 15;23(22):14103. doi: 10.3390/ijms232214103. Int J Mol Sci. 2022. PMID: 36430578 Free PMC article. Review. - Characterization of Signal Sequences Determining the Nuclear/Nucleolar Import and Nuclear Export of the Caprine Arthritis-Encephalitis Virus Rev Protein.
Labrecque M, Marchand C, Archambault D. Labrecque M, et al. Viruses. 2020 Aug 17;12(8):900. doi: 10.3390/v12080900. Viruses. 2020. PMID: 32824614 Free PMC article. - Evidence for a role of spindle matrix formation in cell cycle progression by antibody perturbation.
Yao C, Wang C, Li Y, Zavortink M, Archambault V, Girton J, Johansen KM, Johansen J. Yao C, et al. PLoS One. 2018 Nov 28;13(11):e0208022. doi: 10.1371/journal.pone.0208022. eCollection 2018. PLoS One. 2018. PMID: 30485354 Free PMC article. - Interdependent changes of nuclear lamins, nuclear pore complexes, and ploidy regulate cellular regeneration and stress response in the heart.
Li Y, Bertozzi A, Mann MR, Kühn B. Li Y, et al. Nucleus. 2023 Dec;14(1):2246310. doi: 10.1080/19491034.2023.2246310. Nucleus. 2023. PMID: 37606283 Free PMC article. Review. - Modulation of Cell Identity by Modification of Nuclear Pore Complexes.
Gomar-Alba M, Mendoza M. Gomar-Alba M, et al. Front Genet. 2020 Jan 8;10:1301. doi: 10.3389/fgene.2019.01301. eCollection 2019. Front Genet. 2020. PMID: 31969901 Free PMC article. Review.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. - PubMed
- Allen NP, Huang L, Burlingame A, Rexach M. Proteomic analysis of nucleoporin interacting proteins. J Biol Chem. 2001;276:29268–29274. - PubMed
- Allen TD, Cronshaw JM, Bagley S, Kiseleva E, Goldberg MW. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J Cell Sci. 2000;113(Pt 10):1651–1659. - PubMed
- Amlacher S, Sarges P, Flemming D, van Noort V, Kunze R, Devos DP, Arumugam M, Bork P, Hurt E. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell. 2011;146:277–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources