An injectable nanoparticle generator enhances delivery of cancer therapeutics - PubMed (original) (raw)
doi: 10.1038/nbt.3506. Epub 2016 Mar 14.
Guodong Zhang 1, Junhua Mai 1, Xiaoyong Deng 1, Victor Segura-Ibarra 1, Suhong Wu 1, Jianliang Shen 1, Haoran Liu 1, Zhenhua Hu 1, Lingxiao Chen 1, Yi Huang 1, Eugene Koay 1 3, Yu Huang 1, Jun Liu 4, Joe E Ensor 5, Elvin Blanco 1, Xuewu Liu 1, Mauro Ferrari 1 6, Haifa Shen 1 7
Affiliations
- PMID: 26974511
- PMCID: PMC5070674
- DOI: 10.1038/nbt.3506
An injectable nanoparticle generator enhances delivery of cancer therapeutics
Rong Xu et al. Nat Biotechnol. 2016 Apr.
Abstract
The efficacy of cancer drugs is often limited because only a small fraction of the administered dose accumulates in tumors. Here we report an injectable nanoparticle generator (iNPG) that overcomes multiple biological barriers to cancer drug delivery. The iNPG is a discoidal micrometer-sized particle that can be loaded with chemotherapeutics. We conjugate doxorubicin to poly(L-glutamic acid) by means of a pH-sensitive cleavable linker, and load the polymeric drug (pDox) into iNPG to assemble iNPG-pDox. Once released from iNPG, pDox spontaneously forms nanometer-sized particles in aqueous solution. Intravenously injected iNPG-pDox accumulates at tumors due to natural tropism and enhanced vascular dynamics and releases pDox nanoparticles that are internalized by tumor cells. Intracellularly, pDox nanoparticles are transported to the perinuclear region and cleaved into Dox, thereby avoiding excretion by drug efflux pumps. Compared to its individual components or current therapeutic formulations, iNPG-pDox shows enhanced efficacy in MDA-MB-231 and 4T1 mouse models of metastatic breast cancer, including functional cures in 40-50% of treated mice.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Figure 1. iNPG-pDox characterization and pDox assembly and release from iNPG
(a) Schematic diagram depicting iNPG-pDox composition, pDox prodrug encapsulation, and pDox nanoparticle assembly and release from nanopores. (b) Z-series confocal microscopy imaging of the iNPG-pDox particles, highlighting the presence of pDox (red) within the nanopores of the silicon carrier particle (gray). Scale bar: 1 µm. (c) Three dimensional reconstruction following sagittal cross-sectioning of the iNPG-pDox particles, depicting pDox (red) within the nanopores of the silicon carrier particle (gray), as well as the presence of pDox nanoparticles (red) released from the microparticles. Scale bar: 1 µm. (d) AFM analysis of size distribution of pDox nanoparticles released from iNPG-pDox at pH 7.4. (e) Cryogenic TEM of pDox nanoparticles released from iNPG-pDox at pH 7.4. Scale bar: 150 nm. (f) Release of pDox or disassembled Dox from iNPG-pDox at pH 7.4 and pH 5.2 in 10% FBS. (g) Gel permeation chromatography (GPC) analysis on released pDox and disseminated Dox from iNPG-pDox. pDox was the predominant form at pH 7.4, whereas Dox was released from the polymer at pH 5.2. The inset represents release of Dox in different pH conditions following cleavage from pDox.
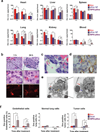
Figure 2. Accumulation of iNPG-pDox in metastatic MDA-MB-231 tumors
(a) Time-dependent tissue biodistribution based on Dox content in mice with MDA-MB-231 lung metastasis after administration of 6 mg/kg Dox, pDox NP, or iNPG-pDox. Differences and significance between iNPG-pDox and Dox or pDox NP were estimated by F-tests with Hommel’s adjustment of multiplicity. (b) iNPG-pDox accumulation in lung metastatic tumors by H&E staining (upper and middle panels). Presence of doxorubicin inside iNPG was visualized under fluorescence microscopy (bottom panel). Scale bar: 10 µm. (c) H&E histological evaluation of tumor nodules, demonstrating co-localization of iNPG-pDox with red blood cells and attachment on tumor microvessels. The red arrow points to an iNPG particle, and blue arrow points to red blood cells. The scale bar represents 5 µm. (d) CD31 staining for tumor microvessels (brown). The red arrow points to an iNPG particles. The scale bar represents 5 µm. (e) TEM analysis of iNPG attachment to microvessel walls in tumor-bearing lung tissues collected 24 h after iNPG-pDox treatment. The red arrow points to an iNPG particle, the yellow arrow points to a tumor cell, and the blue arrow points to a red blood cell inside the vessel. Scale bar: 2 µm. (f) Flow cytometry analysis of Dox distribution in CD31+ endothelial cells, HLA-ABC+ tumor cells, and HLA-ABC−/CD45−/CD31− normal lung cells isolated from post-treatment mice. *: p<0.05; **: p<0.01
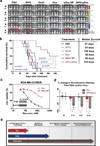
Figure 3. In vivo growth inhibition of lung metastatic MDA-MB-231 tumors, as well as a multidrug resistant cell line, following iNPG-pDox treatment
(a) Bioluminescence monitoring of MDA-MB-231 tumor metastasis in the lung. Nude mice were inoculated with MDA-MB-231 cells carrying a luciferase gene, divided into 6 treatment groups (n = 10 mice/group), and treated weekly with 3 mg/kg Dox or biweekly with 6 mg/kg Doxil, pDox, or iNPG-pDox for 6 weeks. Mice were maintained further thereafter to monitor survival. Images of 5 mice/group are shown. (b) Kaplan-Meier plot of animal survival with median survival time listed in the table. Differences in survival were evaluated by the log-rank test. A global test demonstrated a difference exists among the groups. Pairwise comparisons were performed to evaluate the advantage of iNPG-pDox formulation over the clinical formulations. (c) MTT cell viability assay of MDA-MB-231/MDR cells treated with Dox or pDox NP for 72 h. The inset represents Western blot analysis of P-gp expression in the parental MDA-MB-231 cells and cells transfected with a plasmid carrying the MDR1 gene. (d) Tumor growth in the lung, as measured based on bioluminescence and compared to that of the PBS control group, of mice inoculated with MDA-MB-231/MDR cells carrying a luciferase gene following biweekly administration of PBS, Dox, or iNPG-pDox at a dosage of 6 mg/kg. F-tests of the simple effects were applied to compare the effects of Dox and iNPG-pDox treatments at each time period. (e) Schematic diagram demonstrating the individual components of the iNPG-pDox construct, and the distinct biological barriers that each component is capable of overcoming following systemic administration.
Comment in
- Multistage Delivery Technologies: Multifunctional, Interdisciplinary Approaches to Nanomedicine.
Haynes MT, Huang L. Haynes MT, et al. Mol Ther. 2016 May;24(5):849-51. doi: 10.1038/mt.2016.75. Mol Ther. 2016. PMID: 27198852 Free PMC article. No abstract available.
Similar articles
- Doxorubicin-loaded amphiphilic polypeptide-based nanoparticles as an efficient drug delivery system for cancer therapy.
Lv S, Li M, Tang Z, Song W, Sun H, Liu H, Chen X. Lv S, et al. Acta Biomater. 2013 Dec;9(12):9330-42. doi: 10.1016/j.actbio.2013.08.015. Epub 2013 Aug 17. Acta Biomater. 2013. PMID: 23958784 - Role of integrated cancer nanomedicine in overcoming drug resistance.
Iyer AK, Singh A, Ganta S, Amiji MM. Iyer AK, et al. Adv Drug Deliv Rev. 2013 Nov;65(13-14):1784-802. doi: 10.1016/j.addr.2013.07.012. Epub 2013 Jul 21. Adv Drug Deliv Rev. 2013. PMID: 23880506 Review. - Biodegradable, polymeric nanoparticle delivery systems for cancer therapy.
Pridgen EM, Langer R, Farokhzad OC. Pridgen EM, et al. Nanomedicine (Lond). 2007 Oct;2(5):669-80. doi: 10.2217/17435889.2.5.669. Nanomedicine (Lond). 2007. PMID: 17976029 Review.
Cited by
- Sequential deconstruction of composite drug transport in metastatic breast cancer.
Goel S, Zhang G, Dogra P, Nizzero S, Cristini V, Wang Z, Hu Z, Li Z, Liu X, Shen H, Ferrari M. Goel S, et al. Sci Adv. 2020 Jun 24;6(26):eaba4498. doi: 10.1126/sciadv.aba4498. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32637609 Free PMC article. - Strategies for improving drug delivery: nanocarriers and microenvironmental priming.
Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, Wolfram J. Khalid A, et al. Expert Opin Drug Deliv. 2017 Jul;14(7):865-877. doi: 10.1080/17425247.2017.1243527. Epub 2016 Oct 11. Expert Opin Drug Deliv. 2017. PMID: 27690153 Free PMC article. Review. - Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy.
Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X. Yong T, et al. Nat Commun. 2019 Aug 23;10(1):3838. doi: 10.1038/s41467-019-11718-4. Nat Commun. 2019. PMID: 31444335 Free PMC article. - Nanotherapeutics for Treatment of Pulmonary Arterial Hypertension.
Segura-Ibarra V, Wu S, Hassan N, Moran-Guerrero JA, Ferrari M, Guha A, Karmouty-Quintana H, Blanco E. Segura-Ibarra V, et al. Front Physiol. 2018 Jul 13;9:890. doi: 10.3389/fphys.2018.00890. eCollection 2018. Front Physiol. 2018. PMID: 30061840 Free PMC article. Review. - Global trends in nanomedicine research on triple negative breast cancer: a bibliometric analysis.
Teles RHG, Moralles HF, Cominetti MR. Teles RHG, et al. Int J Nanomedicine. 2018 Apr 17;13:2321-2336. doi: 10.2147/IJN.S164355. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29713164 Free PMC article. Review.
References
- Lien MY, et al. Safety and efficacy of pegylated liposomal doxorubicin-based adjuvant chemotherapy in patients with stage I–III triple-negative breast cancer. Anticancer Res. 2014;34:7319–7326. - PubMed
- Verma S, Dent S, Chow BJ, Rayson D, Safra T. Metastatic breast cancer: the role of pegylated liposomal doxorubicin after conventional anthracyclines. Cancer Treat Rev. 2008;34:391–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA193880/CA/NCI NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- U54CA151668/CA/NCI NIH HHS/United States
- U54 CA143837/CA/NCI NIH HHS/United States
- NIH U54CA143837/CA/NCI NIH HHS/United States
- 1R01CA193880-01A1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous