The future of cancer treatment: immunomodulation, CARs and combination immunotherapy - PubMed (original) (raw)
Review
The future of cancer treatment: immunomodulation, CARs and combination immunotherapy
Danny N Khalil et al. Nat Rev Clin Oncol. 2016 May.
Erratum in
- The future of cancer treatment: immunomodulation, CARs and combination immunotherapy.
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. Khalil DN, et al. Nat Rev Clin Oncol. 2016 Jun;13(6):394. doi: 10.1038/nrclinonc.2016.65. Epub 2016 Apr 26. Nat Rev Clin Oncol. 2016. PMID: 27118494 Free PMC article. No abstract available.
Abstract
In the past decade, advances in the use of monoclonal antibodies (mAbs) and adoptive cellular therapy to treat cancer by modulating the immune response have led to unprecedented responses in patients with advanced-stage tumours that would otherwise have been fatal. To date, three immune-checkpoint-blocking mAbs have been approved in the USA for the treatment of patients with several types of cancer, and more patients will benefit from immunomodulatory mAb therapy in the months and years ahead. Concurrently, the adoptive transfer of genetically modified lymphocytes to treat patients with haematological malignancies has yielded dramatic results, and we anticipate that this approach will rapidly become the standard of care for an increasing number of patients. In this Review, we highlight the latest advances in immunotherapy and discuss the role that it will have in the future of cancer treatment, including settings for which testing combination strategies and 'armoured' CAR T cells are recommended.
Conflict of interest statement
Competing interests statement
D.N.K. and E.L.S. and declare no competing interests. R.J.B. is a co-founder, stockholder, and consultant for Juno Therapeutics Inc. J.D.W. is a consultant for Bristol Myers Squibb, Genentech, Medimmune, Merck Pharmaceuticals and Polynoma Pharmaceuticals.
Figures
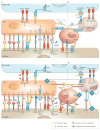
Figure 1. Immunomodulatory monoclonal antibodies and armoured chimeric antigen receptor (CAR) T cells overcome immune suppression
a | Overview of the immune inhibitory molecules that compromise endogenous T–cell antitumour activity. T cells are susceptible to immune inhibitory factors associated within the microenvironment that prevent their full antitumour activity. Such factors include cell surface proteins (such as programmed cell death 1 ligands 1 and 2 (PD-L1 and PD-L2)) and cytokines (such as TGF-β and IL-10). Regulatory T (TREG) cells are representative of inhibitory cellular components of the tumour microenvironment (TME), which also include myeloid-derived suppressor cells, tumour-associated macrophages and other cell types non-depicted. b | Similarly to endogenous T cells, CAR T cells are susceptible to immune inhibitory factors present in the TME. c | Immunomodulatory monoclonal antibodies can be used to overcome local immunosuppression by either activating stimulatory receptors (such as TNFRSF9 (4-1BB) or OX40), or blocking suppressive receptors (for example, programmed cell-death 1 (PD-1) or cytotoxic T-lymphocyte antigen 4 (CTLA-4)). d | Armoured CAR T cells are engineered to express proteins that overcome immunosuppression associated with the TME (such as CD40L, IL-12 or TNFSF9 (4-1BBL)). Ag, antigen; APC, antigen-presenting cell; CD40L, CD40 ligand; Cy, cyclophosphamide; DC, dendritic cell; Flu, fludarabine; GITR, glucocorticoid-induced TNFR family related protein; GITRL, GITR ligand; HMGB1, high mobility group 1 protein; LAG-3, lymphocyte activation gene-3; PSer, phosphatidyl serine; scFv, single-chain variable fragment; TCR, T-cell receptor; TIGIT, T-cell immunoreceptor with immunoglobulin and ITIM domains; TIL, tumour -nfiltrating lymphocyte; TIM-3, T cell immunoglobulin and mucin domain 3.
Figure 2. Neoantigen presentation in the tumour microenvironment
a | Tumour cells can avoid elimination by immunoediting because of low neoantigen presentation (neoAglow). This occurs as a result of a low level of somatic mutations, or because tumour cells might downregulate antigen processing or presentation. NeoAglow tumours might escape detection by endogenous CTLs early in tumorigenesis, and therefore would expand without the pressure to co-evolve within an immunosuppressive microenvironment. This is the ideal setting for CAR T cells to direct a robust antitumour response. b | Tumours that present a high neoantigen burden (neoAghigh) can be eliminated during the immunoediting process (far right). Alternatively, neoAghigh tumour cells might co-evolve within an immunosuppressive tumour microenvironment (TME) that mediates avoidance of T-cell surveillance. This setting is the ideal situation for immune-modulating therapies with monoclonal antibodies to direct a robust antitumour response. c | Tumour types can be ranked by their relative presence of somatic mutations, and this classification can be used to indirectly estimate neoantigen burden. Tumours can be stratified on the basis of somatic mutation prevalence; tumour types with the most robust clinical responses to CAR T-cell therapy (red) or immune modulating mAbs (blue) are stratified by somatic mutation prevalence. ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukaemia; CTL, cytotoxic T cell; mAb, monoclonal antibody; MDSC, myeloid-derived suppressor cell; neoAg, neoantigen; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; TAA, tumour-associated antigen; TAM, tumour-associated macrophage; TCR, T-cell receptor; TIL, tumour infiltrating lymphocyte; TREG cell, regulatory T cell. Image reproduced from Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013),
Similar articles
- Armed T cells with CAR for cancer immunotherapy.
Wei YQ. Wei YQ. Sci China Life Sci. 2016 Apr;59(4):331-2. doi: 10.1007/s11427-016-5047-0. Epub 2016 Apr 15. Sci China Life Sci. 2016. PMID: 27080712 No abstract available. - Treatment of solid tumors with chimeric antigen receptor-engineered T cells: current status and future prospects.
Di S, Li Z. Di S, et al. Sci China Life Sci. 2016 Apr;59(4):360-9. doi: 10.1007/s11427-016-5025-6. Epub 2016 Mar 11. Sci China Life Sci. 2016. PMID: 26968709 Review. - Establishing guidelines for CAR-T cells: challenges and considerations.
Wang W, Qin DY, Zhang BL, Wei W, Wang YS, Wei YQ. Wang W, et al. Sci China Life Sci. 2016 Apr;59(4):333-9. doi: 10.1007/s11427-016-5026-5. Epub 2016 Mar 11. Sci China Life Sci. 2016. PMID: 26965523 Review. - CAR T-cell Therapy: A New Era in Cancer Immunotherapy.
Miliotou AN, Papadopoulou LC. Miliotou AN, et al. Curr Pharm Biotechnol. 2018;19(1):5-18. doi: 10.2174/1389201019666180418095526. Curr Pharm Biotechnol. 2018. PMID: 29667553 Review. - Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors.
Zhang BL, Qin DY, Mo ZM, Li Y, Wei W, Wang YS, Wang W, Wei YQ. Zhang BL, et al. Sci China Life Sci. 2016 Apr;59(4):340-8. doi: 10.1007/s11427-016-5027-4. Epub 2016 Mar 11. Sci China Life Sci. 2016. PMID: 26965525 Review.
Cited by
- Cuproptosis-related lncRNAs emerge as a novel signature for predicting prognosis in prostate carcinoma and functional experimental validation.
Lu Y, Wu J, Li X, Leng Q, Tan J, Huang H, Zhong R, Chen Z, Zhang Y. Lu Y, et al. Front Immunol. 2024 Oct 28;15:1471198. doi: 10.3389/fimmu.2024.1471198. eCollection 2024. Front Immunol. 2024. PMID: 39530098 Free PMC article. - Blood Cell Membrane-Coated Nanomaterials as a Versatile Biomimetic Nanoplatform for Antitumor Applications.
Shen H, Ouyang Y, Zhang L, Li J, Wang S. Shen H, et al. Nanomaterials (Basel). 2024 Oct 31;14(21):1757. doi: 10.3390/nano14211757. Nanomaterials (Basel). 2024. PMID: 39513837 Free PMC article. Review. - Application of Carbon Nanomaterials to Enhancing Tumor Immunotherapy: Current Advances and Prospects.
Li Y, Xu Z, Qi Z, Huang X, Li M, Liu S, Yan Y, Gao M. Li Y, et al. Int J Nanomedicine. 2024 Oct 26;19:10899-10915. doi: 10.2147/IJN.S480799. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39479174 Free PMC article. Review. - The roles of patient-derived xenograft models and artificial intelligence toward precision medicine.
Janitri V, ArulJothi KN, Ravi Mythili VM, Singh SK, Prasher P, Gupta G, Dua K, Hanumanthappa R, Karthikeyan K, Anand K. Janitri V, et al. MedComm (2020). 2024 Sep 25;5(10):e745. doi: 10.1002/mco2.745. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39329017 Free PMC article. Review. - The diagnostic value of histogram analysis of DWI and DKI for the mismatch repair status of rectal adenocarcinoma.
Chen H, Jin Z, Dai X, Zhu J, Chen G. Chen H, et al. Heliyon. 2024 Sep 5;10(18):e37526. doi: 10.1016/j.heliyon.2024.e37526. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309916 Free PMC article.
References
- Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2014;372:320–330. - PubMed
- Robert C, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. - PubMed
- Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA138738/CA/NCI NIH HHS/United States
- R01CA138738‑05/CA/NCI NIH HHS/United States
- P01CA059350/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P01 CA190174/CA/NCI NIH HHS/United States
- P01 CA059350/CA/NCI NIH HHS/United States
- P01CA190174‑01/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous